Label: FIORE RX PIXIE DUST PINK ANTIFUNGAL NAIL POLISH- undecylenic acid film
-
Contains inactivated NDC Code(s)
NDC Code(s): 52261-0204-0 - Packager: Cosco International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 19, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WARNINGS
-
KEEP OUT OF REACH OF CHILDREN
Do not use on children under 2 years of age unless directed by a doctor.
KEEP THIS AND ALL MEDICATION OUT OF REACH OF CHILDREN.
In case of accidental ingestion, contact a physician, emergency medical care facility or poison control center immediately for advice.
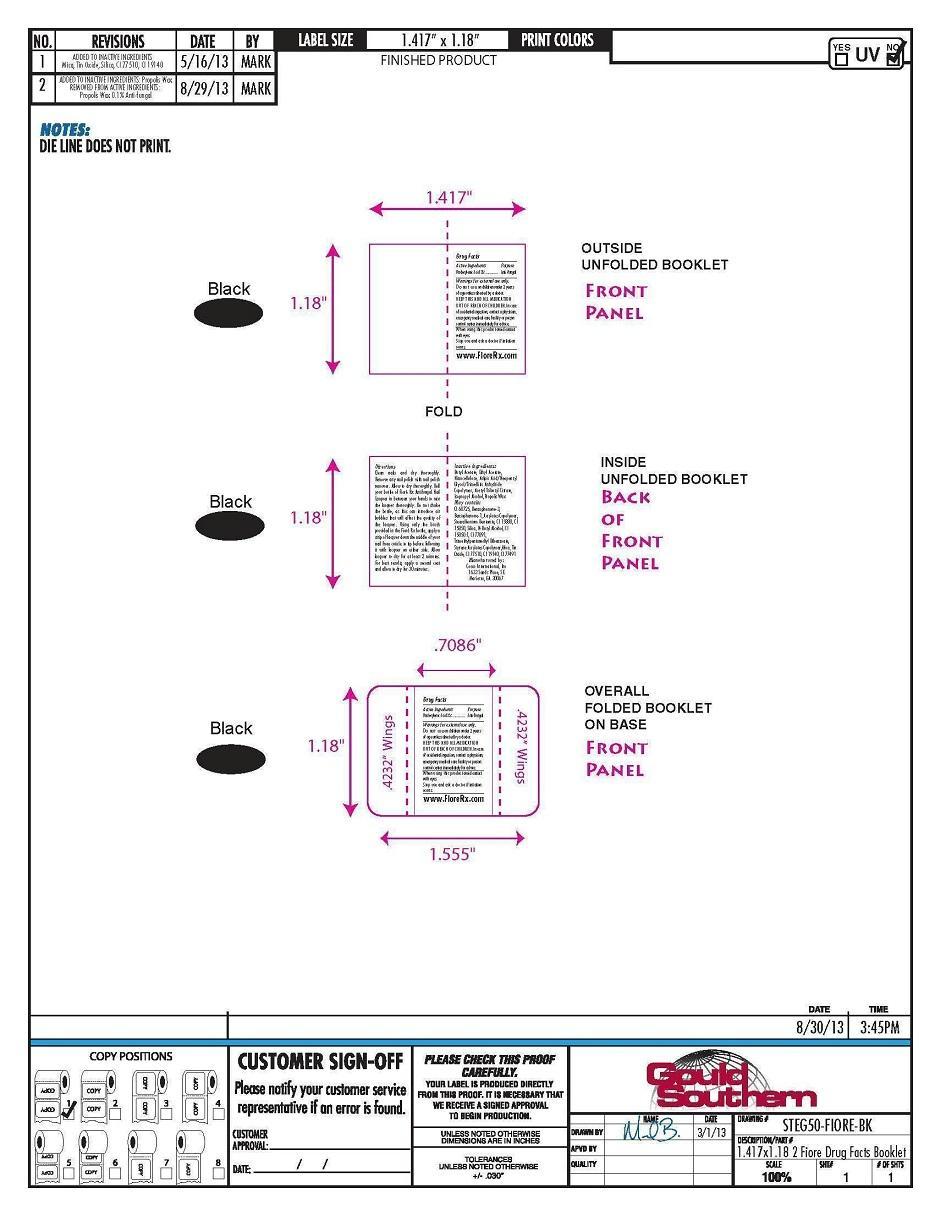
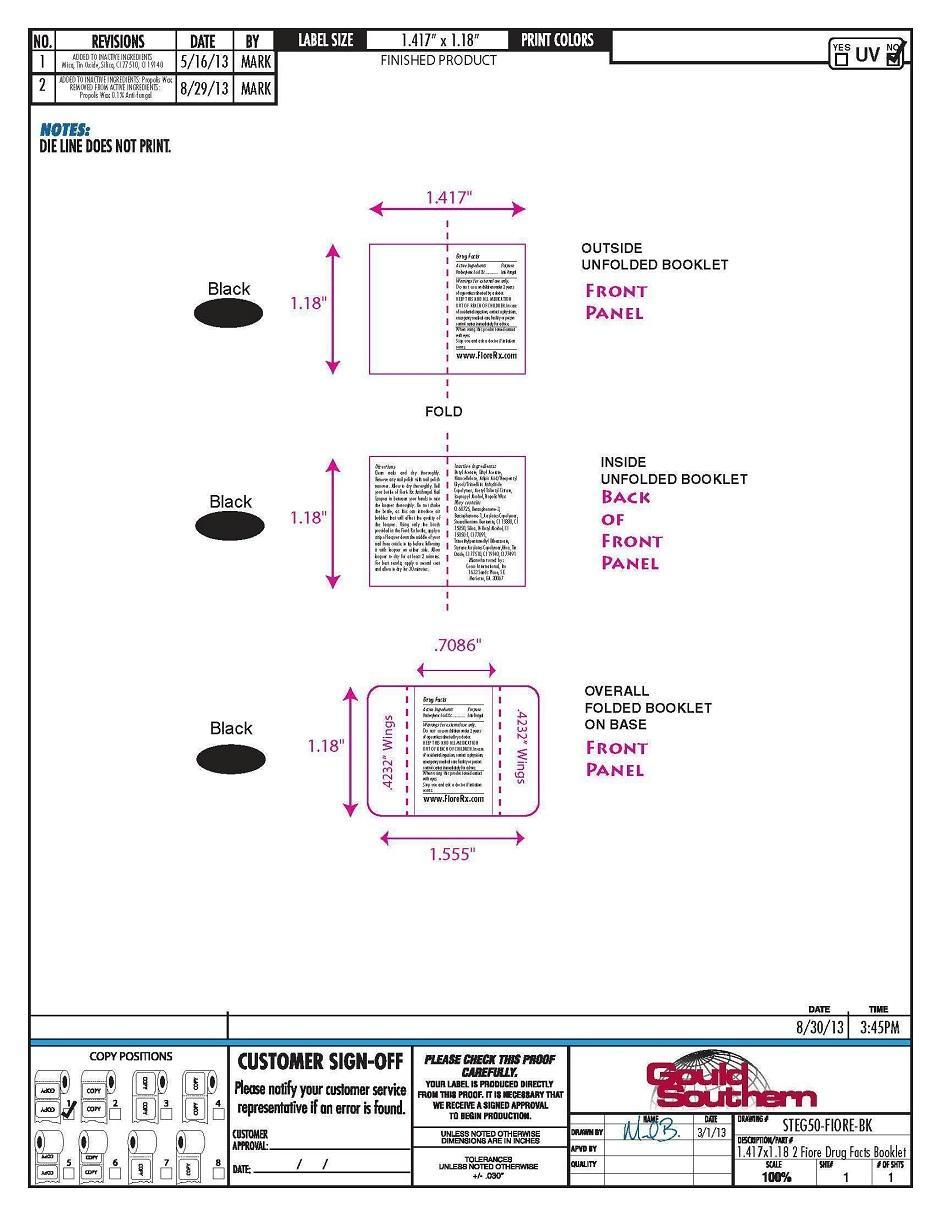
Directions
Clean nails and dry thoroughly.
Remove any nail polish with nail
polish remover. Allow to dry
thoroughly. Roll your bottle of Fioré
Rx Antifungal Nail Lacquer in between
your hands to mix the lacquer
thoroughly. Do not shake the bottle,
as this can introduce air bubbles that
will affect the quality of the lacquer.
Using only the brush provided in the
Fioré Rx bottle, apply a strip of
lacquer down the middle of your nail
from cuticle to tip before following it
with lacquer on either side. Allow
lacquer to dry for at least 2 minutes.
For best results, apply a second coat
and allow to dry for 30 minutes.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FIORE RX PIXIE DUST PINK ANTIFUNGAL NAIL POLISH
undecylenic acid filmProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52261-0204 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Undecylenic Acid (UNII: K3D86KJ24N) (Undecylenic Acid - UNII:K3D86KJ24N) Undecylenic Acid 0.45 g in 15 mL Inactive Ingredients Ingredient Name Strength Butyl Acetate (UNII: 464P5N1905) 6.2260095 g in 15 mL Ethyl Acetate (UNII: 76845O8NMZ) 2.527011 g in 15 mL Pyroxylin (UNII: KYR8BR2X6O) 1.979754 g in 15 mL POLYESTER-10 (UNII: 212N9O2MMZ) 1.41411015 g in 15 mL Acetyltributyl Citrate (UNII: 0ZBX0N59RZ) 0.98987715 g in 15 mL Isopropyl Alcohol (UNII: ND2M416302) 0.84846615 mL in 15 mL DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) 0.141411 g in 15 mL Bentoquatam (UNII: 7F465U79Q1) 0.1272684 g in 15 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.0973554 g in 15 mL BUTYL ALCOHOL (UNII: 8PJ61P6TS3) 0.07071285 g in 15 mL Silicon Dioxide (UNII: ETJ7Z6XBU4) 0.04242765 g in 15 mL Benzoresorcinol (UNII: LJ54R4Z029) 0.02828505 g in 15 mL MICA (UNII: V8A1AW0880) 0.0267153 g in 15 mL PROPOLIS WAX (UNII: 6Y8XYV2NOF) 0.015 g in 15 mL Trimethylpentanediyl Dibenzoate (UNII: Y8PB83G67A) 0.0141426 g in 15 mL D&C RED NO. 34 (UNII: BAN556989E) 0.0007122 g in 15 mL D&C RED NO. 6 (UNII: 481744AI4O) 0.00037785 g in 15 mL STANNIC OXIDE (UNII: KM7N50LOS6) 0.00036345 g in 15 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52261-0204-0 15 mL in 1 BOTTLE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 05/21/2013 Labeler - Cosco International, Inc. (016433141) Registrant - Cosco International, Inc. (016433141) Establishment Name Address ID/FEI Business Operations Cosco International, Inc. 016433141 manufacture(52261-0204) , label(52261-0204) , pack(52261-0204)