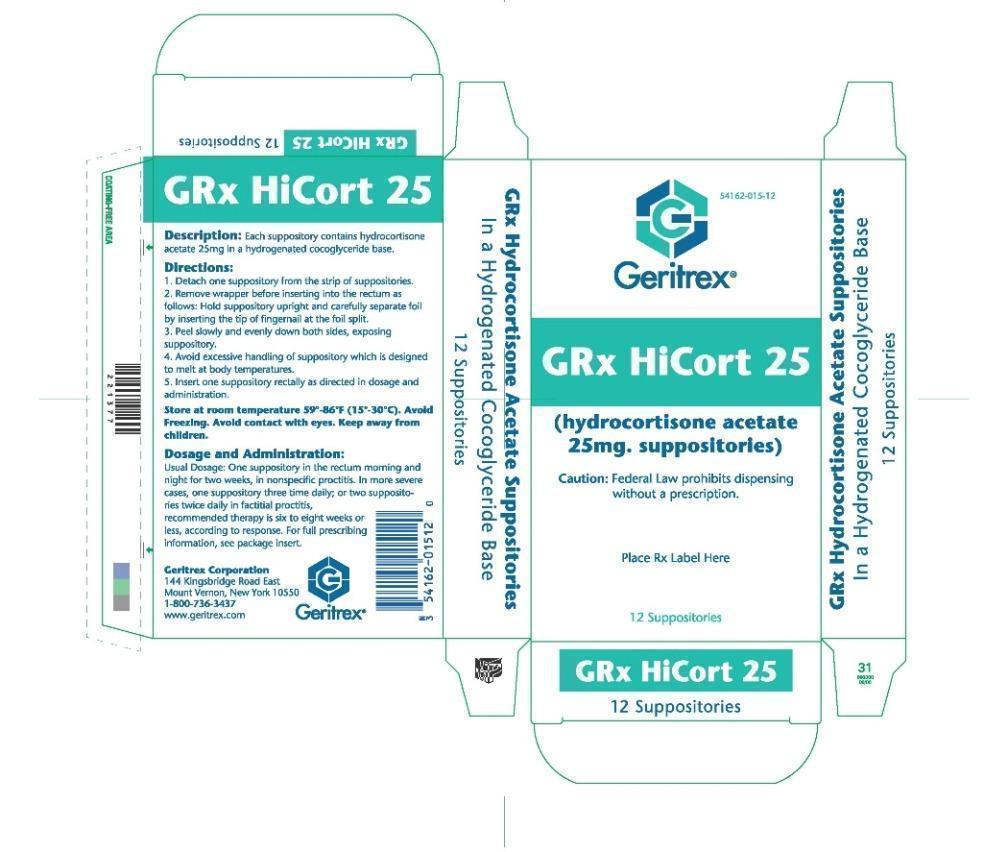
GRX HICORT- hydrocortisone acetate suppository
GERITREX CORP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
GRx HiCort 25
Usage
In inflamed hemrrhoids, post irradiation (factitial) proctitis as an adjunct in the treatement of chronic ulcerative colitis, cryptitis and other
inflammatory condition of anorectum and pruritus ani
Directions
Detach one suppository from the strip of suppositories
Remove wrapper before inserting into the rectum as follows hold suppository upright and carefully separate foil by inserting the tips of fingernail
at the foil split
Peel slowly and evenly down both sides, exposing suppository
Avoid excessive handling of suppository which is designed to melt at body temperatures
Insert one suppository rectally as directed in dosage and administration
Dosage and Administraion
One suppository in the rectum morning and night for two week, in nonspecific proctitis In more severe cases, one suppository three time daily
or two suppository twice daily in factitial proctitis, recommended therapy is six to eight weeks or less, according to response. For full prescribing information, see package insert
| GRX HICORT
hydrocortisone acetate suppository |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - GERITREX CORP (112796248) |
| Registrant - GERITREX CORP (112796248) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GERITREX CORP | 112796248 | manufacture(54162-015) | |