Label: ANEW AGE-TRANSFORMING FOUNDATION- octinoxate, titanium dioxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 10096-0171-1, 10096-0171-2 - Packager: Avon Products, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 10, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
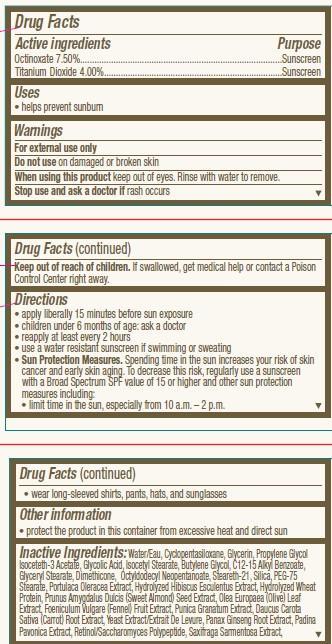
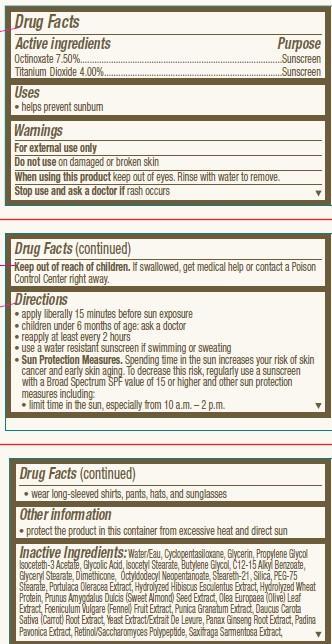
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
• apply liberally 15 minutes before sun exposure
• children under 6 months of age: ask a doctor
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin
cancer and early skin aging. To decrease this risk, regularly use a sunscreen
with a Broad Spectrum SPF value of 15 or higher and other sun protection
measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.• wear long-sleeved shirts, pants, hats, and sunglasses
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive Ingredients:Water/Eau, Cyclopentasiloxane, Glycerin, Propylene Glycol Isoceteth-3 Acetate, Glycolic Acid, Isocetyl Stearate, Butylene Glycol, C12-15 Alkyl Benzoate, Glyceryl Stearate, Dimethicone, Octyldodecyl Neopentanoate, Steareth-21, Silica, PEG-75 Stearate, Portulaca Oleracea Extract, Hydrolyzed Hibiscus Esculentus Extract, Hydrolyzed Wheat Protein, Prunus Amygdalus Dulcis (Sweet Almond) Seed Extract, Olea Europaea (Olive) Leaf Extract, Foeniculum Vulgare (Fennel) Fruit Extract, Punica Granatum Extract, Daucus Carota Sativa (Carrot) Root Extract, Yeast Extract/Extrait De Levure, Panax Ginseng Root Extract, Padina Pavonica Extract, Retinol/Saccharomyces Polypeptide, Saxifraga Sarmentosa Extract, Vitis Vinifera (Grape) Fruit Extract, Aesculus Hippocastanum (Horse Chestnut) Seed Extract, Plankton Extract, Morus Nigra Root Extract, Scutellaria Baicalensis Root Extract, Medicago Sativa (Alfalfa) Extract, Phaeodactylum Tricornutum Extract, Tilia Cordata Wood Extract, Steareth-2, Ammonium Hydroxide, Nylon-12, Sodium Laureth Sulfate, PEG-12 Dimethicone, Magnesium Aluminum Silicate, Parfum/Fragrance, Paraffin, Xanthan Gum, Talc, Xymenynic Acid, Mannitol, Polymethyl Methacrylate, Phytol, Alumina, Glycogen, Oryzanol, PEG-40 Hydrogenated Castor Oil, Caprylic/Capric Triglyceride, Caprylyl Glycol, Calcium Pantothenate, Biotin, Tocopherol, Disodium EDTA, Phenoxyethanol, Methylparaben, Propylparaben. May Contain: Titanium Dioxide/CI 77891, Iron Oxides, Mica/CI 77019, Bismuth Oxychloride/CI 77163.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANEW AGE-TRANSFORMING FOUNDATION
octinoxate, titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10096-0171 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 40 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10096-0171-2 1 in 1 CARTON 1 NDC:10096-0171-1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/09/2013 Labeler - Avon Products, Inc (001468693) Establishment Name Address ID/FEI Business Operations Avon Products, Inc 005149471 manufacture(10096-0171)