Label: HYPERRHO S/D FULL DOSE (rho(d) immune globulin- human solution
- NDC Code(s): 13533-631-02, 13533-631-03, 13533-631-11, 13533-631-20
- Packager: GRIFOLS USA, LLC
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Rho(D) Immune Globulin (Human) — HyperRHO® S/D Full Dose treated with solvent/detergent is a colorless to pale yellow or pink sterile solution of immune globulin containing antibodies to Rho(D) for intramuscular administration; it is preservative-free, in a latex-free delivery system. HyperRHO S/D Full Dose is prepared by cold ethanol fractionation from human plasma. The immune globulin is isolated from solubilized Cohn fraction II. The fraction II solution is adjusted to a final concentration of 0.3% tri-n-butyl phosphate (TNBP) and 0.2% sodium cholate. After the addition of solvent (TNBP) and detergent (sodium cholate), the solution is heated to 30°C and maintained at that temperature for not less than 6 hours. After the viral inactivation step, the reactants are removed by precipitation, filtration and finally ultrafiltration and diafiltration. HyperRHO S/D Full Dose is formulated as a 15–18% protein solution at a pH of 6.4–7.2 in 0.21–0.32 M glycine. HyperRHO S/D Full Dose is then incubated in the final container for 21–28 days at 20–27°C.
The potency is equal to or greater than 1500 IU (300 mcg). Each single dose syringe contains sufficient anti-Rho(D) to effectively suppress the immunizing potential of 15 mL of Rho(D) positive red blood cells.(2-4)
The removal and inactivation of spiked model enveloped and non-enveloped viruses during the manufacturing process for HyperRHO S/D Full Dose has been validated in laboratory studies. Human Immunodeficiency Virus, Type 1 (HIV-1), was chosen as the relevant virus for blood products; Bovine Viral Diarrhea Virus (BVDV) was chosen to model Hepatitis C virus; Pseudorabies virus (PRV) was chosen to model Human Herpes viruses and other large enveloped DNA viruses; and Reo virus type 3 (Reo) was chosen to model non-enveloped viruses and for its resistance to physical and chemical inactivation. Significant removal of model enveloped and non-enveloped viruses is achieved at two steps in the Cohn fractionation process leading to the collection of Cohn Fraction II: the precipitation and removal of Fraction III in the processing of Fraction II + IIIW suspension to Effluent III and the filtration step in the processing of Effluent III to Filtrate III. Significant inactivation of enveloped viruses is achieved at the time of treatment of solubilized Cohn Fraction II with TNBP/sodium cholate.
Additionally, the manufacturing process was investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the vCJD and CJD agents.(18-21)
Studies of the HyperRHO S/D manufacturing process demonstrate that TSE clearance is achieved during the Pooled Plasma to Effluent III Fractionation Process (6.7 log10). These studies provide reasonable assurance that low levels of CJD/vCJD agent infectivity, if present in the starting material, would be removed.
-
CLINICAL PHARMACOLOGY
HyperRHO S/D Full Dose is used to prevent isoimmunization in the Rho(D) negative individual exposed to Rho(D) positive blood as a result of a fetomaternal hemorrhage occurring during a delivery of an Rho(D) positive infant, abortion (either spontaneous or induced), or following amniocentesis or abdominal trauma. Similarly, immunization resulting in the production of anti-Rho(D) following transfusion of Rh positive red cells to an Rho(D) negative recipient may be prevented by administering Rho(D) Immune Globulin (Human).(5,6)
Rh hemolytic disease of the newborn is the result of the active immunization of an Rho(D) negative mother by Rho(D) positive red cells entering the maternal circulation during a previous delivery, abortion, amniocentesis, abdominal trauma, or as a result of red cell transfusion.(7,8) HyperRHO S/D Full Dose acts by suppressing the immune response of Rho(D) negative individuals to Rho(D) positive red blood cells. The mechanism of action of HyperRHO S/D Full Dose is not fully understood.
The administration of Rho(D) Immune Globulin (Human) within 72 hours of a full-term delivery of an Rho(D) positive infant by an Rho(D) negative mother reduces the incidence of Rh isoimmunization from 12%–13% to 1%–2%.(9)
The 1%–2% treatment failures are probably due to isoimmunization occurring during the latter part of pregnancy or following delivery.(10) Bowman and Pollock(11) have reported that the incidence of isoimmunization can be further reduced from approximately 1.6% to less than 0.1% by administering Rho(D) Immune Globulin (Human) in two doses, one antenatal at 28 weeks’ gestation and another following delivery.
In a clinical study in eight healthy human adults receiving another hyperimmune immune globulin product treated with solvent/detergent, Rabies Immune Globulin (Human), HyperRAB® S/D, prepared by the same manufacturing process, detectable passive antibody titers were observed in the serum of all subjects by 24 hours post injection and persisted through the 21 day study period. These results suggest that passive immunization with immune globulin products is not affected by the solvent/detergent treatment.
-
INDICATIONS AND USAGE
Pregnancy and Other Obstetric Conditions
HyperRHO S/D Full Dose is recommended for the prevention of Rh hemolytic disease of the newborn by its administration to the Rho(D) negative mother within 72 hours after birth of an Rho(D) positive infant,(12) providing the following criteria are met:
-
The mother must be Rho(D) negative and must not already be sensitized to the Rho(D) factor.
-
Her child must be Rho(D) positive, and should have a negative direct antiglobulin test (see PRECAUTIONS).
If HyperRHO S/D Full Dose is administered antepartum, it is essential that the mother receive another dose of HyperRHO S/D Full Dose after delivery of an Rho(D) positive infant.
If the father can be determined to be Rho(D) negative, HyperRHO S/D Full Dose need not be given.
HyperRHO S/D Full Dose should be administered within 72 hours to all nonimmunized Rho(D) negative women who have undergone spontaneous or induced abortion, following ruptured tubal pregnancy, amniocentesis or abdominal trauma unless the blood group of the fetus or the father is known to be Rho(D) negative.(7,8) If the fetal blood group cannot be determined, one must assume that it is Rho(D) positive,(2) and HyperRHO S/D Full Dose should be administered to the mother.
Transfusion
HyperRHO S/D Full Dose may be used to prevent isoimmunization in Rho(D) negative individuals who have been transfused with Rho(D) positive red blood cells or blood components containing red blood cells.(5,13)
-
- CONTRAINDICATIONS
-
WARNINGS
HyperRHO S/D Full Dose is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, and, theoretically, the Creutzfeldt-Jakob Disease (CJD) agent that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses. Despite these measures, such products can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in such products. Individuals who receive infusions of blood or plasma products may develop signs and/or symptoms of some viral infections, particularly hepatitis C. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Grifols Therapeutics LLC [1-800-520-2807].
The physician should discuss the risks and benefits of this product with the patient, before prescribing or administering it to the patient.
NEVER ADMINISTER HYPERRHO S/D FULL DOSE INTRAVENOUSLY. INJECT ONLY INTRAMUSCULARLY. NEVER ADMINISTER TO THE NEONATE.
Rho(D) Immune Globulin (Human) should be given with caution to patients with a history of prior systemic allergic reactions following the administration of human immunoglobulin preparations.
The attending physician who wishes to administer Rho(D) Immune Globulin (Human) to persons with isolated immunoglobulin A (IgA) deficiency must weigh the benefits of immunization against the potential risks of hypersensitivity reactions. Such persons have increased potential for developing antibodies to IgA and could have anaphylactic reactions to subsequent administration of blood products that contain IgA.
As with all preparations administered by the intramuscular route, bleeding complications may be encountered in patients with thrombocytopenia or other bleeding disorders.
-
PRECAUTIONS
General
A large fetomaternal hemorrhage late in pregnancy or following delivery may cause a weak mixed field positive Du test result. If there is any doubt about the mother’s Rh type, she should be given Rho(D) Immune Globulin (Human). A screening test to detect fetal red blood cells may be helpful in such cases.
If more than 15 mL of D-positive fetal red blood cells are present in the mother’s circulation, more than a single dose of HyperRHO S/D Full Dose is required. Failure to recognize this may result in the administration of an inadequate dose.
Although systemic reactions to human immunoglobulin preparations are rare, epinephrine should be available for treatment of acute anaphylactic reactions.
Drug Interactions
Other antibodies in the Rho(D) Immune Globulin (Human) preparation may interfere with the response to live vaccines such as measles, mumps, polio or rubella. Therefore, immunization with live vaccines should not be given within 3 months after Rho(D) Immune Globulin (Human) administration.
Drug/Laboratory Interactions
Babies born of women given Rho(D) Immune Globulin (Human) antepartum may have a weakly positive direct antiglobulin test at birth.
Passively acquired anti-Rho(D) may be detected in maternal serum if antibody screening tests are performed subsequent to antepartum or postpartum administration of Rho(D) Immune Globulin (Human).
Pregnancy
Animal reproduction studies have not been conducted with Rho(D) Immune Globulin (Human) — HyperRHO® S/D Full Dose. It is also not known whether HyperRHO S/D Full Dose can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. HyperRHO S/D Full Dose should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
Reactions to Rho(D) Immune Globulin (Human) are infrequent in Rho(D) negative individuals and consist primarily of slight soreness at the site of injection and slight temperature elevation. While sensitization to repeated injections of human immune globulin is extremely rare, it has occurred. Elevated bilirubin levels have been reported in some individuals receiving multiple doses of Rho(D) Immune Globulin (Human) following mismatched transfusions. This is believed to be due to a relatively rapid rate of foreign red cell destruction.
-
DOSAGE AND ADMINISTRATION
NEVER ADMINISTER HYPERRHO S/D FULL DOSE INTRAVENOUSLY. INJECT ONLY INTRAMUSCULARLY. NEVER ADMINISTER TO THE NEONATE.
Pregnancy and Other Obstetric Conditions
-
For postpartum prophylaxis, administer one syringe of HyperRHO S/D Full Dose (1500 IU; 300 mcg), preferably within 72 hours of delivery. Although a lesser degree of protection is afforded if Rh antibody is administered beyond the 72-hour period, HyperRHO S/D Full Dose may still be given.(7,14]) Full-term deliveries can vary in their dosage requirements depending on the magnitude of the fetomaternal hemorrhage. One full dose syringe of HyperRHO S/D Full Dose provides sufficient antibody to prevent Rh sensitization if the volume of red blood cells that has entered the circulation is 15 mL or less.(2-4) In instances where a large (greater than 30 mL of whole blood or 15 mL red blood cells) fetomaternal hemorrhage is suspected, a fetal red cell count by an approved laboratory technique (e.g., modified Kleihauer-Betke acid elution stain technique) should be performed to determine the dosage of immune globulin required.(8,15) The red blood cell volume of the calculated fetomaternal hemorrhage is divided by 15 mL to obtain the number of syringes of HyperRHO S/D Full Dose (1500 IU; 300 mcg) for administration.(3,8,13) If more than 15 mL of red cells is suspected or if the dose calculation results in a fraction, administer the next higher whole number of syringes (e.g., if 1.4, give 2 syringes).
-
For antenatal prophylaxis, one full dose syringe of HyperRHO S/D Full Dose (1500 IU; 300 mcg) is administered at approximately 28 weeks’ gestation. This must be followed by another full dose (1500 IU; 300 mcg) , preferably within 72 hours following delivery, if the infant is Rh positive.
-
Following threatened abortion at any stage of gestation with continuation of pregnancy, it is recommended that a full dose of HyperRHO S/D Full Dose (1500 IU; 300 mcg) be given. If more than 15 mL of red cells is suspected due to fetomaternal hemorrhage, the same dose modification in No. 1 above applies.
-
Following miscarriage, abortion, or termination of ectopic pregnancy at or beyond 13 weeks’ gestation, it is recommended that a HyperRHO S/D Full Dose (1500 IU; 300 mcg) be given. If more than 15 mL of red cells is suspected due to fetomaternal hemorrhage, the same dose modification in No. 1 above applies. If pregnancy is terminated prior to 13 weeks’ gestation, where licensed, a single dose of HyperRHO® S/D Mini-Dose (250 IU; 50mcg) may be used instead of HyperRHO S/D Full Dose (1500 IU; 300 mcg).
-
Following amniocentesis at either 15 to 18 weeks’ gestation or during the third trimester, or following abdominal trauma in the second or third trimester, it is recommended that a HyperRHO S/D Full Dose (1500 IU; 300 mcg) be administered. If there is a fetomaternal hemorrhage in excess of 15 mL of red cells, the same dose modification in No. 1 applies.
If abdominal trauma, amniocentesis, or other adverse event requires the administration of HyperRHO S/D Full Dose (1500 IU; 300 mcg) at 13 to 18 weeks’ gestation, another full dose should be given at 26 to 28 weeks. To maintain protection throughout pregnancy, the level of passively acquired anti-Rho(D) should not be allowed to fall below the level required to prevent an immune response to Rh positive red cells. The half-life of IgG is 23 to 26 days. In any case, a HyperRHO S/D Full Dose should be given within 72 hours after delivery if the baby is Rh positive. If delivery occurs within 3 weeks after the last dose, the postpartum dose may be withheld unless there is a fetomaternal hemorrhage in excess of 15 mL of red blood cells.(16)
Transfusion
In the case of a transfusion of Rho(D) positive red cells to an Rho(D) negative recipient, the volume of Rh positive whole blood administered is multiplied by the hematocrit of the donor unit giving the volume of red blood cells transfused. The volume of red blood cells is divided by 15 mL which provides the number of syringes of HyperRHO S/D Full Dose to be administered.
If the dose calculated results in a fraction, the next higher whole number of syringes should be administered (e.g., if 1.4, give 2 syringes). HyperRHO S/D Full Dose should be administered within 72 hours after an incompatible transfusion, but preferably as soon as possible.
Injection Procedure
DO NOT INJECT INTRAVENOUSLY. DO NOT INJECT NEONATE. HyperRHO S/D Full Dose is administered intramuscularly, preferably in the deltoid muscle of the upper arm or lateral thigh muscle. The gluteal region should not be used as an injection site because of the risk of injury to the sciatic nerve.(17)
- A.
- Single Syringe Dose
- INJECT ENTIRE CONTENTS OF THE SYRINGE INTO THE INDIVIDUAL INTRAMUSCULARLY.
- B.
- Multiple Syringe Dose
-
- Calculate the number of syringes of HyperRHO S/D Full Dose to be given (see Dosage section).
-
The total volume of HyperRHO S/D Full Dose can be given in divided doses at different sites at one time or the total dose may be divided and injected at intervals, provided the total dosage is given within 72 hours of the fetomaternal hemorrhage or transfusion. USING STERILE TECHNIQUE, INJECT THE ENTIRE CONTENTS OF THE CALCULATED NUMBER OF SYRINGES INTRAMUSCULARLY INTO THE PATIENT.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HyperRHO S/D Full Dose is supplied with a syringe and an attached UltraSafe® Needle Guard for your protection and convenience. Please follow instructions below for proper use of syringe and UltraSafe® Needle Guard.
Directions for Syringe Usage
- Remove the prefilled syringe from the package. Lift syringe by barrel, not by plunger.
- Twist the plunger rod clockwise until the threads are seated.
- With the rubber needle shield secured on the syringe tip, push the plunger rod forward a few millimeters to break any friction seal between the rubber stopper and the glass syringe barrel.
-
Remove the needle shield and expel air bubbles. [Do not remove the rubber needle shield to prepare the product for administration until immediately prior to the anticipated injection time.]
- Proceed with hypodermic needle puncture.
- Aspirate prior to injection to confirm that the needle is not in a vein or artery.
- Inject the medication.
-
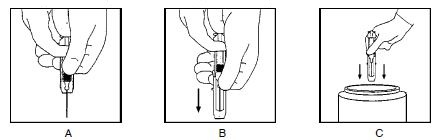
Keeping your hands behind the needle, grasp the guard with free hand and slide forward toward needle until it is completely covered and guard clicks into place. If audible click is not heard, guard may not be completely activated. (See Diagrams A and B)
-
Place entire prefilled glass syringe with guard activated into an approved sharps container for proper disposal. (See Diagram C)
A number of factors could reduce the efficacy of this product or even result in an ill effect following its use. These include improper storage and handling of the product after it leaves our hands, diagnosis, dosage, method of administration, and biological differences in individual patients. Because of these factors, it is important that this product be stored properly and that the directions be followed carefully during use.
-
-
HOW SUPPLIED
HyperRHO S/D Full Dose is available in a single dose syringe with attached needle.
HyperRHO S/D Full Dose is packaged as 1 syringe per carton, and as 10 cartons, each with a single dose disposable syringe. HyperRHO S/D Full Dose is preservative-free, in a latex-free delivery system.
NDC Number Size 13533-631-02 Syringe 13533-631-11 Syringe (10 Pack) - STORAGE
- CAUTION
-
REFERENCES
-
Gunson HH, Bowell PJ, Kirkwood TBL: Collaborative study to recalibrate the International Reference Preparation of Anti-D Immunoglobulin. J Clin Pathol 33:249-53, 1980.
-
Rho(D) immune globulin (human). Med Lett Drugs Ther 16(1):3-4, 1974.
-
Pollack W, Ascari WQ, Kochesky RJ, et al: Studies on Rh prophylaxis. I. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion 11(6):333-9, 1971.
-
Unpublished data on file.
-
Pollack W, Ascari WQ, Crispen JF, et al: Studies on Rh prophylaxis. II. Rh immune prophylaxis after transfusion with Rh-positive blood. Transfusion 11(6):340-4, 1971.
-
Keith LG, Houser GH: Anti-Rh immune globulin after a massive transfusion accident. Transfusion 11(3):176, 1971.
-
The selective use of Rho(D) immune globulin (RhIG). ACOG Tech Bull 61, 1981.
-
Current uses of Rho immune globulin and detection of antibodies. ACOG Tech Bull 35, 1976.
-
Pollack W: Rh hemolytic disease of the newborn: its cause and prevention. Prog Clin Biol Res 70:185-203, 1981.
-
Bowman JM, Chown B, Lewis M, et al: Rh isoimmunization during pregnancy: antenatal prophylaxis. Can Med Assoc J 118(6):623-7, 1978.
-
Bowman JM, Pollock JM: Antenatal prophylaxis of Rh isoimmunization: 28-weeks’- gestation service program. Can Med Assoc J 118(6):627-30, 1978.
-
Ascari WQ, Allen AE, Baker WJ, et al: Rho(D) immune globulin (human): evaluation in women at risk of Rh immunization. JAMA 205(1):1-4, 1968.
-
Prevention of Rh sensitization. WHO Tech Rep Ser 468:25, 1971.
-
Samson D, Mollison PL: Effect on primary Rh immunization of delayed administration of anti-Rh. Immunology 28:349-57, 1975.
-
Finn R, Harper DT, Stallings SA, et al: Transplacental hemorrhage. Transfusion 3(2):114-24, 1963.
-
Garraty G (ed.): Hemolytic disease of the newborn. Arlington, VA, American Association of Blood Banks, 1984, p 78.
-
Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP): General recommendations on immunization. MMWR 2002: 51(RR02), 1-36.
-
Stenland CJ, Lee DC, Brown P, et al: Partitioning of human and sheep forms of the pathogenic prion protein during the purification of therapeutic proteins from human plasma. Transfusion 2002. 42(11):1497-500.
-
Lee DC, Stenland CJ, Miller JL, et al: A direct relationship between the partitioning of the pathogenic prion protein and transmissible spongiform encephalopathy infectivity during the purification of plasma proteins. Transfusion 2001. 41(4):449-55.
-
Lee DC, Stenland CJ, Hartwell RC, et al: Monitoring plasma processing steps with a sensitive Western blot assay for the detection of the prion protein. J Virol Methods 2000. 84(1):77-89.
-
Cai K, Miller JL, Stenland CJ, et al: Solvent-dependent precipitation of prion protein. Biochim Biophys Acta 2002. 1597(1):28-35.
Rev. 6/2018
GRIFOLS
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
3052584
-
-
The Rh Factor and Your Pregnancy
Information About Pregnancy Protection
The Rh Factor and When It Is Important

The Rh factor is one of many blood group antigens found on the surface of red blood cells. If you have this antigen you are considered Rh positive. If you don’t, then you are considered Rh negative. Everyone is either Rh positive or Rh negative. One type is neither better nor worse than the other, only different.
Your Rh factor is important if you are an Rh negative woman and you become pregnant, or if you receive a blood transfusion.
How the Rh Factor Can Affect Your Future
If you have Rh negative blood, there are two situations that can affect you:
- 1.
- If the father of your baby is Rh positive, the baby will probably be Rh positive too. An Rh negative woman carrying an Rh positive baby may have an immune reaction if some of the baby’s Rh positive blood cells enter her bloodstream.
-
This immune reaction, called isoimmunization, means your body’s defense system recognizes Rh positive blood as foreign from your own and produces “antibodies” to destroy the invading Rh positive blood cells. -
The passage of blood from the baby to the mother’s bloodstream happens most often at delivery, but can also occur during miscarriage, the termination of pregnancy, amniocentesis (test performed to determine fetal health), or due to an injury or trauma. It is important to note that a small number of women develop antibodies to Rh positive blood cells during pregnancy for no apparent reason. -
Antibodies to Rh positive blood may not be a problem in first pregnancies; however, the antibodies stay in your bloodstream, ready to attack invading Rh positive blood cells, for many years to come. This can lead to problems in future pregnancies by causing miscarriage or a disease known as hemolytic disease of the newborn. -
Babies born to Rh positive mothers, regardless of the father’s blood type, will usually be free of the dangers of hemolytic disease.
- 2.
- Someday it may become necessary for you to receive a blood transfusion. If Rh positive antibodies already reside in your bloodstream due to isoimmunization and the blood you receive is Rh positive due to error or lifesaving reasons, your Rh positive antibodies will become mobilized and destroy the donor Rh positive cells. As a result, the transfusion could be unsuccessful and possibly harmful to you.
Hemolytic Disease of the Newborn: A Threat to Your Baby
When an Rh negative woman has Rh positive antibodies in her blood and the baby she is carrying is Rh positive, the antibodies could possibly enter the baby’s bloodstream, attack the baby’s red blood cells and cause hemolytic disease of the newborn. At birth, the infant suffering from hemolytic disease may be jaundiced and anemic or suffer permanent damage of the brain and central nervous system which may result in mental retardation, hearing loss, or cerebral palsy. Extensive medical care can be required, including an exchange transfusion, in which all of the baby’s blood is replaced. This usually stops the destruction of the baby’s red blood cells and gives the infant a chance to survive.
The risk of hemolytic disease of the newborn is slight with the first baby, but increases with each successive pregnancy.
Preventing Hemolytic Disease
HyperRHO® S/D, Rho(D) Immune Globulin (Human) can prevent hemolytic disease of the newborn, provided Rh positive antibodies do not already reside in your bloodstream.
HyperRHO S/D is a specially prepared gamma globulin with a high level of preformed antibodies against Rh positive blood cells. The injection of HyperRHO S/D destroys any Rh positive blood cells that may have entered the mother’s bloodstream and prevents the mother’s immune system from producing Rh positive antibodies; thus protecting the baby from developing hemolytic disease.
HyperRHO S/D Full Dose — When Prescribed
Pregnancy and Other Obstetric Conditions Pertaining to Rh Negative Women
HyperRHO S/D Full Dose (1500 IU; 300 mcg) is administered during pregnancy if you fall into a high-risk category. For example, you are at risk of producing Rh positive antibodies if you have an amniocentesis procedure performed, or if you have a miscarriage or other termination of pregnancy at or beyond 13 weeks' gestation.
Laboratory findings have shown that some Rh negative women develop Rh positive antibodies during the last weeks of pregnancy even without an antibody-stimulating event. As a preventive measure, your physician will probably recommend the first injection of HyperRHO S/D Full Dose at the 28th week of pregnancy.
In both of the above situations, if the blood type of the father or baby can be determined to be Rh negative, an injection of HyperRHO S/D is not required.
Another injection of HyperRHO S/D Full Dose is administered within 72 hours of delivery of an Rh positive baby.
Blood Transfusion
HyperRHO S/D Full Dose (1500 IU; 300 mcg) may be used to prevent isoimmunization in Rh negative individuals who have been transfused with Rh positive red blood cells or blood components containing red blood cells.
HyperRHO S/D Mini-Dose — When Prescribed
A single dose of HyperRHO S/D Mini-Dose (250 IU; 50 mcg) may be prescribed for an Rh negative woman instead of HyperRHO S/D Full Dose in the event of miscarriage or other termination of pregnancy occurring prior to 13 weeks' gestation. HyperRHO S/D Mini-Dose is not required if the blood type of the father or fetus can be determined to be Rh negative.
Will You Need HyperRHO S/D Again?
HyperRHO S/D provides protection only if you have not already produced Rh positive antibodies. Women who have developed antibodies through previous pregnancy, miscarriage, other termination of pregnancy, or blood transfusion cannot be protected by HyperRHO S/D. This is why with each pregnancy it is important to have HyperRHO S/D injections within the prescribed time period.
Reactions to HyperRHO S/D
You may feel a temporary soreness at the site of the injection. You may also have a slight and temporary change in body temperature. In very rare instances, an allergic type of reaction can occur, for which your physician will take appropriate measures.
Delivering a Sound, Healthy Baby
Your physician can answer any questions you may have about receiving a HyperRHO S/D injection to prevent hemolytic disease of the newborn. If you know that you are Rh negative and you are pregnant, you should discuss your situation with your physician. Today, with HyperRHO S/D, hemolytic disease of the newborn can be reduced to its lowest possible rate of incidence.
GRIFOLS
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
3052584
(Rev. 6/2018)Development of Hemolytic Disease
1
Rh positive (+) father.
Rh negative (–) mother.
2
Pregnancy: Rh– mother is carrying
Rh+ baby.
3.
The passage of Rh+ blood from the baby to the mother's bloodstream happens most often at delivery, but can also occur during miscarriage, other termination of pregnancy, amniocentesis, or due to injury or trauma.
4.
Rh+ antibodies stay in your blood stream, ready to attack invading Rh+ blood cells, for many years to come.
5.
Next pregnancy, mother’s Rh+ antibodies enter baby’s Rh+ bloodstream, attacking baby’s blood cells and causing hemolytic disease of the newborn.
How HyperRHO S/D Immune Globulin Can Prevent Hemolytic Disease
1
You will probably be given two injections of
HyperRHO S/D Full Dose, one at the 28th week of your pregnancy and another within 72 hours of delivery, miscarriage or other termination of pregnancy. A single injection of HyperRHO S/D Mini-Dose may be prescribed instead of HyperRHO
S/D Full Dose in the event of miscarriage or other termination of pregnancy occurring prior to 13 weeks' gestation.
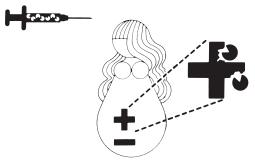
2
HyperRHO S/D immunization prevents formation of mother's own Rh+ antibodies. Mother’s bloodstream remains free of Rh+ antibodies.
3
Next pregnancy, baby develops normally. HyperRHO S/D should be administered following delivery, miscarriage, or other termination of pregnancy to continue protection if baby is Rh+.
-
PACKAGE LABEL
3061239
Lot
Exp.
Rho(D) Immune
Globulin (Human)
HyperRHO® S/D Full Dose
Solvent/Detergent Treated
The patient and physician should discuss
the risks and benefits of this product.
Grifols Therapeutics LLC
RTP, NC 27709 USA
U.S. License No. 1871
1500 IU
1500 IU (300 mcg)
Rho(D)
Immune
Globulin
(Human)
HyperRHO® S/D
Full Dose
Rx only
FOR INTRAMUSCULAR INJECTION ONLY.
DO NOT GIVE INTRAVENOUSLY.
NEVER ADMINISTER TO NEONATES.
NDC 13533-631-02
GRIFOLS
Solvent/Detergent Treated
Contents: One single dose
disposable syringe with attached
needle
Rho(D) Immune Globulin (Human) -
HyperRHO® S/D is a sterile solution
of immunoglobulin containing
15%-18% protein stabilized with
0.21-0.32 M glycine. The pH is
adjusted with sodium carbonate.
The patient and physician should
discuss the risks and benefits of this
product.
The potency of each single dose of
HyperRHO® S/D is equal to or
greater than 1500 IU (300 mcg).
Caution: Rho(D) Immune Globulin
(Human) should be administered
to unsensitized Rh negative
women preferably within 3 days
after any miscarriage or after
delivery of an Rh positive infant.
For complete dosage and
administration information,
read enclosed package insert.
For directions for syringe usage,
see enclosed package insert.
Store at 2–8°C (36–46°F).
Do not freeze.
Do not use if the syringe is
prematurely engaged.
Not returnable for credit or
exchange.
Preservative-free, latex-free
delivery system
Discard unused portion
Grifols Therapeutics LLC
Research Triangle Park,
NC 27709 USA
U.S. License No. 1871
GRIFOLS
Carton: 3061240
NDC 13533-631-02 GTIN 00313533631024
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXXXXXXXX
HyperRHO® S/D
Full Dose
1500 IU (300 mcg)

1500 IU (300 mcg)
NDC 13533-631-11
Rho(D) Immune Globulin (Human)
HyperRHO® S/D Full Dose
Rx only
FOR INTRAMUSCULAR INJECTION ONLY.
DO NOT GIVE INTRAVENOUSLY.
NEVER ADMINISTER TO NEONATES.
GRIFOLS
Solvent/Detergent Treated
Contents: 10 cartons, each with a single dose
disposable syringe with attached needle
Rho(D) Immune Globulin (Human) - HyperRHO® S/D
Full Dose is a sterile solution of immunoglobulin containing
15%-18% protein stabilized with 0.21-0.32 M glycine.
The pH is adjusted with sodium carbonate.
The patient and physician should discuss the risks and
benefits of this product.
The quantity of Rho(D) antibody in each single dose
syringe of HyperRHO® S/D Full Dose is equal to or greater
than 1500 IU (300 mcg).
Caution: Rho(D) Immune Globulin (Human) should be
administered to unsensitized Rh negative women
preferably within 3 days after any miscarriage or after
delivery of an Rh positive infant.
GRIFOLS
For complete dosage and administration information, read
enclosed package insert.
For directions for syringe usage, see enclosed package
insert.
Store at 2-8°C (36-46°F). Do not freeze.
Do not use if the syringe is prematurely engaged.
Not returnable for credit or exchange.
Preservative-free, latex-free delivery system
Discard unused portion.
Grifols Therapeutics LLC
Research Triangle Park,
NC 27709 USA
U.S. License No. 1871
GRIFOLS
1500 IU (300 mcg)
NDC 13533-631-11
Rho(D) Immune Globulin (Human)
HyperRHO® S/D Full Dose
Rx Only
FOR INTRAMUSCULAR INJECTION ONLY.
DO NOT GIVE INTRAVENOUSLY.
NEVER ADMINISTER TO NEONATES.
GRIFOLS
GTIN 00313533631116
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXXXXXXXX
NDC 13533-631-11 1500 IU (300 mcg)
Rho(D) Immune Globulin (Human)
HyperRHO®S/D Full Dose
Solvent/Detergent Treated
Contents: 10 cartons, each with a single dose
disposable syringe with attached needle
Carton: 3061241

-
INGREDIENTS AND APPEARANCE
HYPERRHO S/D FULL DOSE
rho(d) immune globulin (human) solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:13533-631 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Human Rho(d) Immune Globulin (UNII: 48W7181FLP) (Human Rho(d) Immune Globulin - UNII:48W7181FLP) Human Rho(d) Immune Globulin 1500 [iU] Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (YELLOW (clear liquid, colorless to pale yellow or pink)) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13533-631-02 1 in 1 CARTON 1 NDC:13533-631-20 1 in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:13533-631-11 10 in 1 CARTON 2 NDC:13533-631-03 1 in 1 CARTON 2 NDC:13533-631-20 1 in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101141 08/14/1996 Labeler - GRIFOLS USA, LLC (048987452) Establishment Name Address ID/FEI Business Operations GRIFOLS THERAPEUTICS LLC 611019113 manufacture(13533-631)



