Label: SULFACETAMIDE SODIUM lotion
- NDC Code(s): 45802-896-26
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each mL of Sulfacetamide Sodium Topical Suspension USP, 10% (Lotion) contains 100 mg of sulfacetamide sodium in a vehicle consisting of diethanolamine; EDTA; hydroxyethyl cellulose; lauramide DEA; methylparaben; polyethylene glycol 400, monolaurate; propylene glycol; purified water; simethicone; sodium chloride; sodium metabisulfite and xanthan gum.
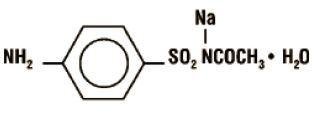
Sulfacetamide sodium is a sulfonamide with antibacterial activity. Chemically, sulfacetamide sodium is N'-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
-
CLINICAL PHARMACOLOGY
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, based on sulfonamides acting as a competitive inhibitor of paraminobenzoic acid (PABA) utilization, an essential component for bacterial growth. While absorption through intact skin in humans has not been determined, in vitro studies with human cadaver skin indicated a percutaneous absorption of about 4%. Sulfacetamide sodium is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine largely unchanged. The biological half-life has been reported to be between 7 to 13 hours.
The pharmacokinetics of sulfacetamide and its major metabolite sulfanilamide in sulfacetamide sodium lotion was evaluated in adult subjects (N=14) with acne vulgaris. The subjects applied sulfacetamide sodium lotion to their face, back, chest and shoulders every 12 hours for 28 days. The percentage of the applied dose of sulfacetamide sodium lotion excreted in the urine as sulfacetamide plus sulfanilamide ranged from 0.08 to 0.33%.
- INDICATIONS
-
CONTRAINDICATIONS
Sulfacetamide Sodium Topical Suspension USP, 10% (Lotion) is contraindicated for use by patients having known hypersensitivity to sulfonamides or any other component of this preparation (see WARNINGS section).
-
WARNINGS
Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Hypersensitivity reactions may occur when a sulfonamide is readministered, irrespective of the route of administration. Sensitivity reactions have been reported in individuals with no prior history of sulfonamide hypersensitivity. At the first sign of hypersensitivity, skin rash or other reactions, discontinue use of this preparation (see ADVERSE REACTIONS section).
Sulfacetamide Sodium Topical Suspension USP, 10% (Lotion) contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people (see CONTRAINDICATIONS section).
-
PRECAUTIONS
General -
For external use only. Keep away from eyes. If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or sensitization during long-term therapy. Hypersensitivity reactions may occur when a sulfonamide is readministered irrespective of the route of administration, and cross-sensitivity between different sulfonamides may occur. Sulfacetamide sodium can cause reddening and scaling of the skin. Particular caution should be employed if areas of involved skin to be treated are denuded or abraded.
Keep out of the reach of children.
Carcinogenesis, Mutagenesis, Impairment of Fertility -
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Pregnancy
Teratogenic Effects: Pregnancy Category C - Animal reproduction studies have not been conducted with Sulfacetamide Sodium Topical Suspension USP, 10% (Lotion). It is also not known whether Sulfacetamide Sodium Topical Suspension USP, 10% (Lotion) can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sulfacetamide Sodium Topical Suspension USP, 10% (Lotion) should be given to a pregnant woman only if clearly needed.
Kernicterus may occur in the newborn as a result of treatment of a pregnant woman at term with orally administered sulfonamide. There are no adequate and well controlled studies of Sulfacetamide Sodium Topical Suspension USP, 10% (Lotion) in pregnant women, and it is not known whether topically applied sulfonamides can cause fetal harm when administered to a pregnant woman.
Nursing Mothers -
It is not known whether sulfacetamide sodium is excreted in the human milk following topical use of Sulfacetamide Sodium Topical Suspension USP, 10% (Lotion). Systemically administered sulfonamides are capable of producing kernicterus in the infants of lactating women. Small amounts of orally administered sulfonamides have been reported to be eliminated in human milk. Because many drugs are excreted in human milk, caution should be exercised in prescribing for nursing women.
-
ADVERSE REACTIONS
In controlled clinical trials for the management of acne vulgaris, the occurrence of adverse reactions associated with the use of sulfacetamide sodium lotion was infrequent and restricted to local events. The total incidence of adverse reactions reported in these studies was less than 2%. Only one of 105 patients treated with sulfacetamide sodium lotion had adverse reactions of erythema, itching and edema. It has been reported that sulfacetamide sodium may cause local irritation, stinging and burning. While the irritation may be transient, occasionally, the use of medication has to be discontinued.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SULFACETAMIDE SODIUM
sulfacetamide sodium lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45802-896 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFACETAMIDE SODIUM (UNII: 4NRT660KJQ) (SULFACETAMIDE - UNII:4965G3J0F5) SULFACETAMIDE SODIUM 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIETHANOLAMINE (UNII: AZE05TDV2V) EDETIC ACID (UNII: 9G34HU7RV0) LAURIC DIETHANOLAMIDE (UNII: I29I2VHG38) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM METABISULFITE (UNII: 4VON5FNS3C) XANTHAN GUM (UNII: TTV12P4NEE) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-896-26 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/17/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078649 10/17/2012 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)