Label: TRAUMACARE- arnica montana capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 63545-517-01 - Packager: Hahnemann Laboratories, Inc. dba Alpine Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 14, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Keep Out of Reach of Children
-
Uses
Reduces pain, bruising, and swelling after major trauma.
TraumaCare is a single complete dosage regimen of Homeopathic Arnica used to speed healing of soft tissue damaged by a major trauma such as an accident, surgery or sports injury. Clinical research has shown patients taking the TraumaCare regimen recover (their bruises and swelling go away) in as little as half the time of patients taking a placebo.
- Warnings
- Drug Interactions
-
Directions
Directions
Adults and children 6 years and older. Take one capsule every 8 hours starting as soon as possible after accident, injury, or surgery. For a planned surgery, take one capsule 1-12 hours prior to surgery, then every 8 hours. Do not eat or drink anything for 15 minutes minutes before and after taking TraumaCare. If symptoms persist see your doctor.
-
Additional Information About Product
What is Arnica?
Homeopathic Arnica Montana - also known simply as "Arnica" - has been used for centuries to reduce the uncomfortable swelling and bruising associated with a physical trauma. Prepared from a daisy-like plant native to the Swiss Alps, the herb is toxic; conversely, the resulting homeopathic medication is not, due to the small doses of Arnica used in Homeopathy.
What is TraumaCare?
TraumaCare is a controlled dosage regimen combining prescription-strength and low-potency Homeopathic Arnica Montana. Clinically proven, TraumaCare goes beyond treating pain - it speeds healing. Recommended by doctors, TraumaCare has demonstrated a reduction in the amount of visible bruising in patients within the first day and even more dramatically one week later.
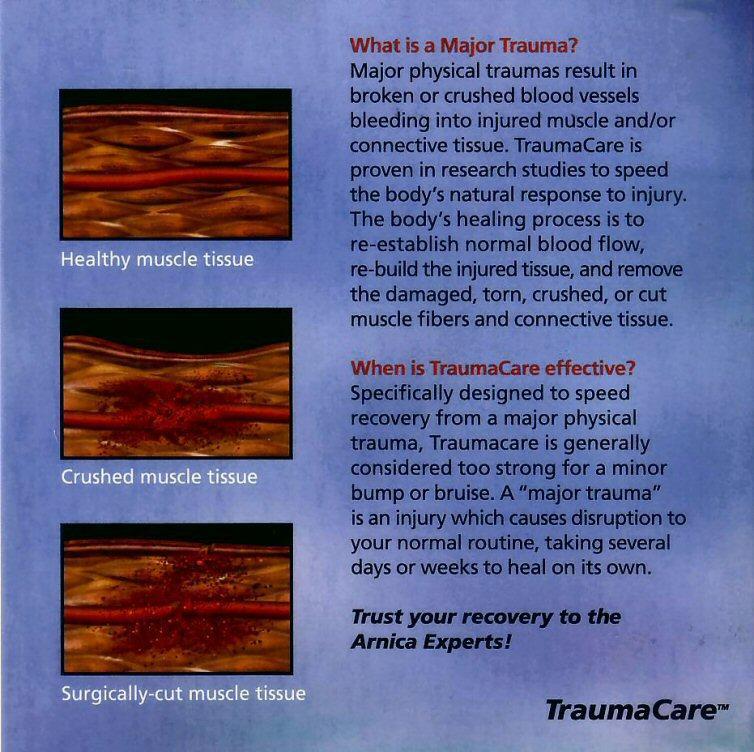
What is a Major Trauma?
Major physical traumas result in broken or crushed blood vessels bleeding into injured muscle and/or connective tissue. TraumaCare is proven in research studies to speed the body's natural response to injury. The body's healing process is to re-establish normal blood flow, re-build the injured tissue, and remove the damaged, torn, crushed, or cut muscle fibers and connective tissue.
When is TraumaCare effective?
Specifically designed to speed recovery from a major physical trauma, Traumacare is generally considered too strong for a minor bump or bruise. A "major trauma" is an injury which causes disruption to your normal routine, taking several days or weeks to heal on its own.
Trust your recovery to the Arnica Experts!
TraumaCare TM
- Inactive Ingredients
-
Package Label
TraumaCare TM Reduces pain, bruising, and major swelling after major trauma
Prescription Strength Homeopathic Arnica
Speeds recovery from Accidents, Sports Injuries, and Surgery
Speeds recovery from major traumas:
- fractures and sprains
- muscle pulls
- facial injuries
- knee injuries
- shoulder injuries
- orthopedic surgery
- oral surgery
- back surgery
- plastic surgery
- general surgery
- Car accidents
- bike accidents
- Basketball
- Baseball
- Football
- Hockey
- Tennis
- Soccer
THIS PRODUCT IS SAFETY SEALED. THE BOX IS SEALED AND THE CAPSULES ARE INDIVIDUALLY BLISTER SEALED.DO NOT USE IF SEALS OR BLISTERS ARE BROKEN.
Printed with soy based inks by a Forest Stewardship Council Certified Printer.
Developed and distributed by Alpine Pharmaceuticals, San Rafael, CA 94901 a wholly owned subsidiary of Hahnemann Laboratories, Inc. All rights reserved.
Lot:TCQ221 Use before Feb 2012 NDC 63545-517-01
A Product of Alpine Pharmaceuticals
Questions or Comments call 888-746-4542 email info@alpinepharma.com or visit online at www.alpinepharma.com
ALL NATURAL 12 capsules For Adults and Children 6 years and older



-
INGREDIENTS AND APPEARANCE
TRAUMACARE
arnica montana capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63545-517 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_M] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE (UNII: J2B2A4N98G) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange (orange/peach) Score no score Shape CAPSULE (capsule) Size 18mm Flavor Imprint Code TraumaCare01 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63545-517-01 12 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/30/2007 Labeler - Hahnemann Laboratories, Inc. dba Alpine Pharmaceuticals (147098081) Registrant - Hahnemann Laboratories, Inc. dba Alpine Pharmaceuticals (147098081) Establishment Name Address ID/FEI Business Operations Hahnemann Laboratories, Inc. dba Alpine Pharmaceuticals 147098081 manufacture(63545-517)



