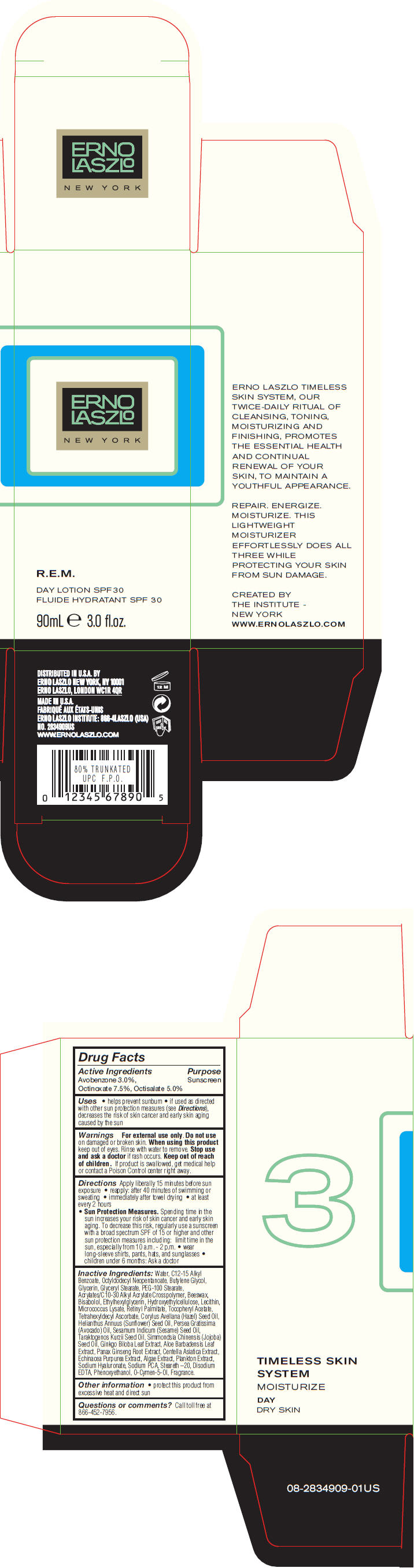
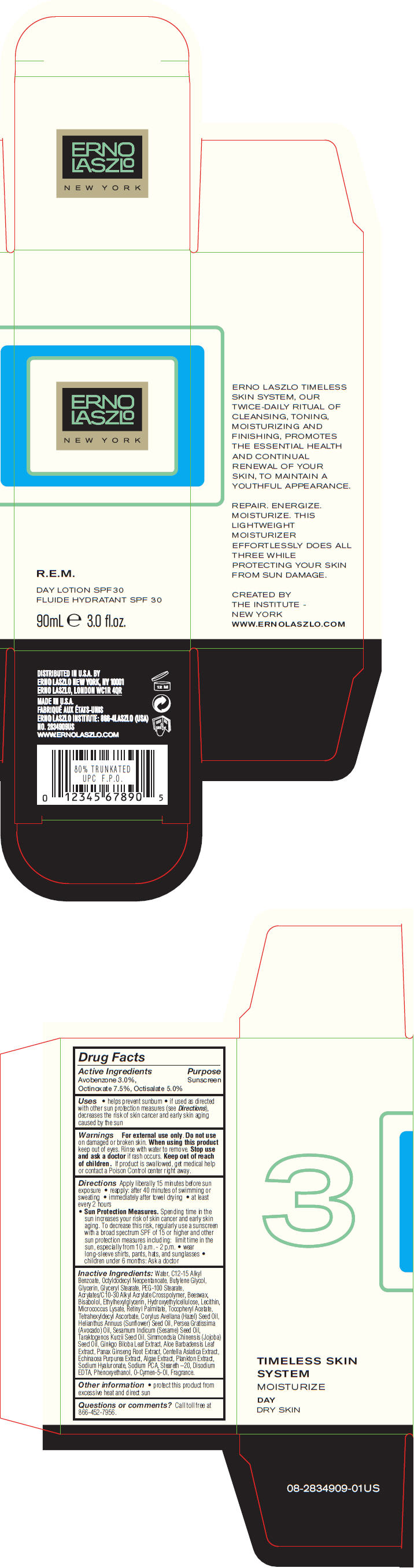
Label: R.E.M. DAY SPF 30- avobenzone, octinoxate, and octisalate lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 57913-2834-9 - Packager: Erno Laszlo, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 10, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
Apply liberally 15 minutes before sun exposure
- reapply: after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
Water, C12-15 Alkyl Benzoate, Octyldodecyl Neopentanoate, Butylene Glycol, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Beeswax, Bisabolol, Ethylhexylglycerin, Hydroxyethylcellulose, Lecithin, Micrococcus Lysate, Retinyl Palmitate, Tocopheryl Acetate, Tetrahexyldecyl Ascorbate, Corylus Avellana (Hazel) Seed Oil, Helianthus Annuus (Sunflower) Seed Oil, Persea Gratissima (Avocado) Oil, Sesamum Indicum (Sesame) Seed Oil, Taraktogenos Kurzii Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Ginkgo Biloba Leaf Extract, Aloe Barbadensis Leaf Extract, Panax Ginseng Root Extract, Centella Asiatica Extract, Echinacea Purpurea Extract, Algae Extract, Plankton Extract, Sodium Hyaluronate, Sodium PCA, Steareth –20, Disodium EDTA, Phenoxyethanol, O-Cymen-5-Ol, Fragrance.
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 90 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
R.E.M. DAY SPF 30
avobenzone, octinoxate, and octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57913-2834 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Octyldodecyl Neopentanoate (UNII: X8725R883T) Butylene Glycol (UNII: 3XUS85K0RA) Glycerin (UNII: PDC6A3C0OX) Glyceryl Monostearate (UNII: 230OU9XXE4) PEG-100 Stearate (UNII: YD01N1999R) Yellow Wax (UNII: 2ZA36H0S2V) Levomenol (UNII: 24WE03BX2T) Ethylhexylglycerin (UNII: 147D247K3P) Vitamin A Palmitate (UNII: 1D1K0N0VVC) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Tetrahexyldecyl Ascorbate (UNII: 9LBV3F07AZ) European Hazelnut Oil (UNII: 8RQ2839AVG) Sunflower Oil (UNII: 3W1JG795YI) Avocado Oil (UNII: 6VNO72PFC1) Sesame Oil (UNII: QX10HYY4QV) Hydnocarpus Kurzii Seed Oil (UNII: N757YEZ18Q) Jojoba Oil (UNII: 724GKU717M) Ginkgo (UNII: 19FUJ2C58T) Aloe Vera Leaf (UNII: ZY81Z83H0X) Asian Ginseng (UNII: CUQ3A77YXI) Centella Asiatica (UNII: 7M867G6T1U) Echinacea Purpurea (UNII: QI7G114Y98) Hyaluronate Sodium (UNII: YSE9PPT4TH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Steareth-20 (UNII: L0Q8IK9E08) Edetate Disodium (UNII: 7FLD91C86K) Phenoxyethanol (UNII: HIE492ZZ3T) O-Cymen-5-Ol (UNII: H41B6Q1I9L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57913-2834-9 1 in 1 CARTON 1 90 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/10/2013 Labeler - Erno Laszlo, Inc. (098821031) Establishment Name Address ID/FEI Business Operations Mana Products 078870292 MANUFACTURE(57913-2834) , LABEL(57913-2834) , PACK(57913-2834)