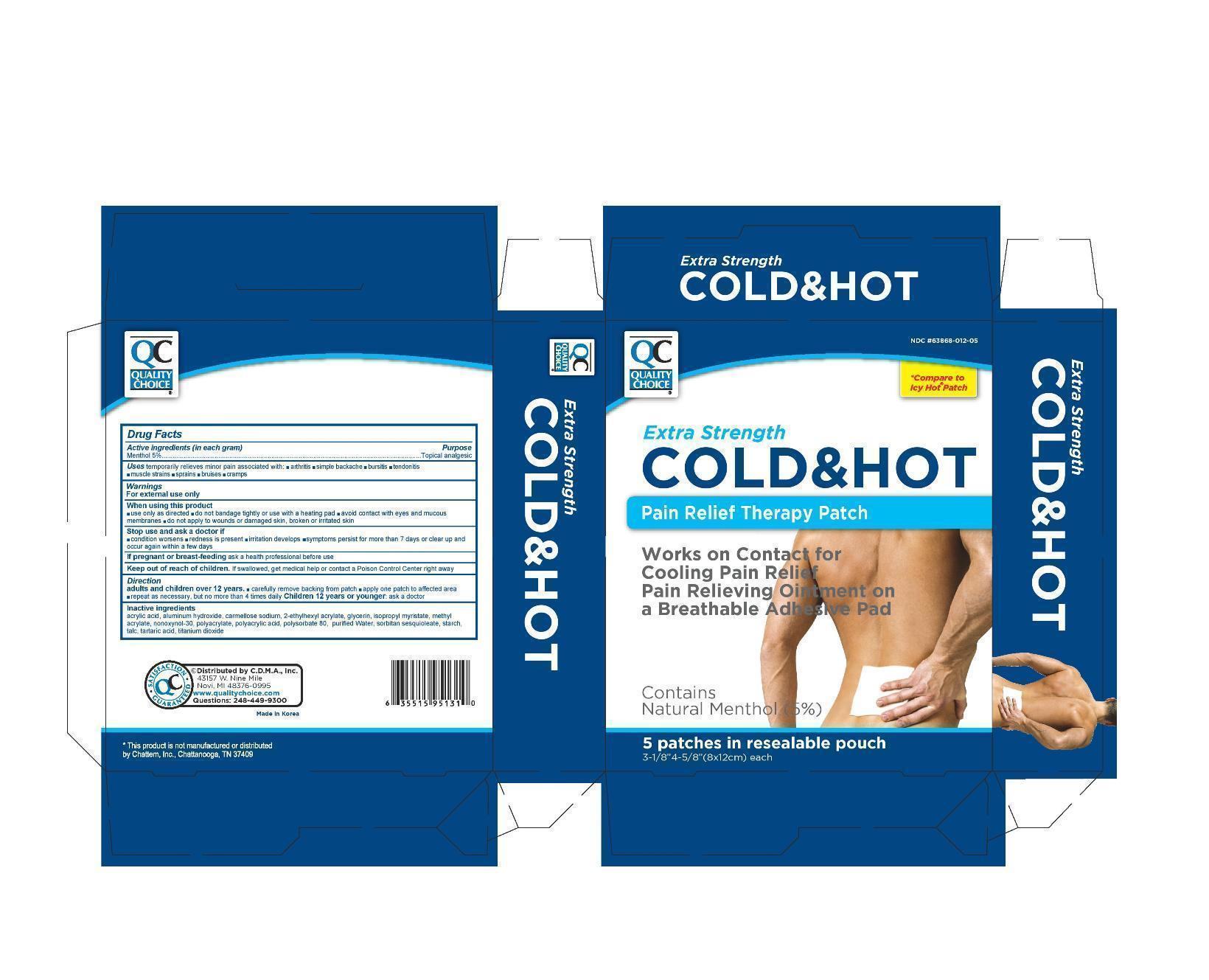
Label: QC COLD AND HOT PAIN RELIEF- menthol patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 63868-012-05 - Packager: CHAIN DRUG MARKETING ASSOCIATION INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 7, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- use only as directed
- avoid contact with eyes and mucous membranes
- do not bandage tightly or use with a heating pad
- do not apply to wounds or damaged skin, broken or irritated skin
- Directions
- Inactive ingredients
- package label
-
INGREDIENTS AND APPEARANCE
QC COLD AND HOT PAIN RELIEF
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 400 mg Inactive Ingredients Ingredient Name Strength ACRYLIC ACID (UNII: J94PBK7X8S) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) METHYL ACRYLATE (UNII: WC487PR91H) NONOXYNOL-30 (UNII: JJX07DG188) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) TALC (UNII: 7SEV7J4R1U) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-012-05 1 in 1 CARTON 1 5 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/12/2011 Labeler - CHAIN DRUG MARKETING ASSOCIATION INC (011920774)