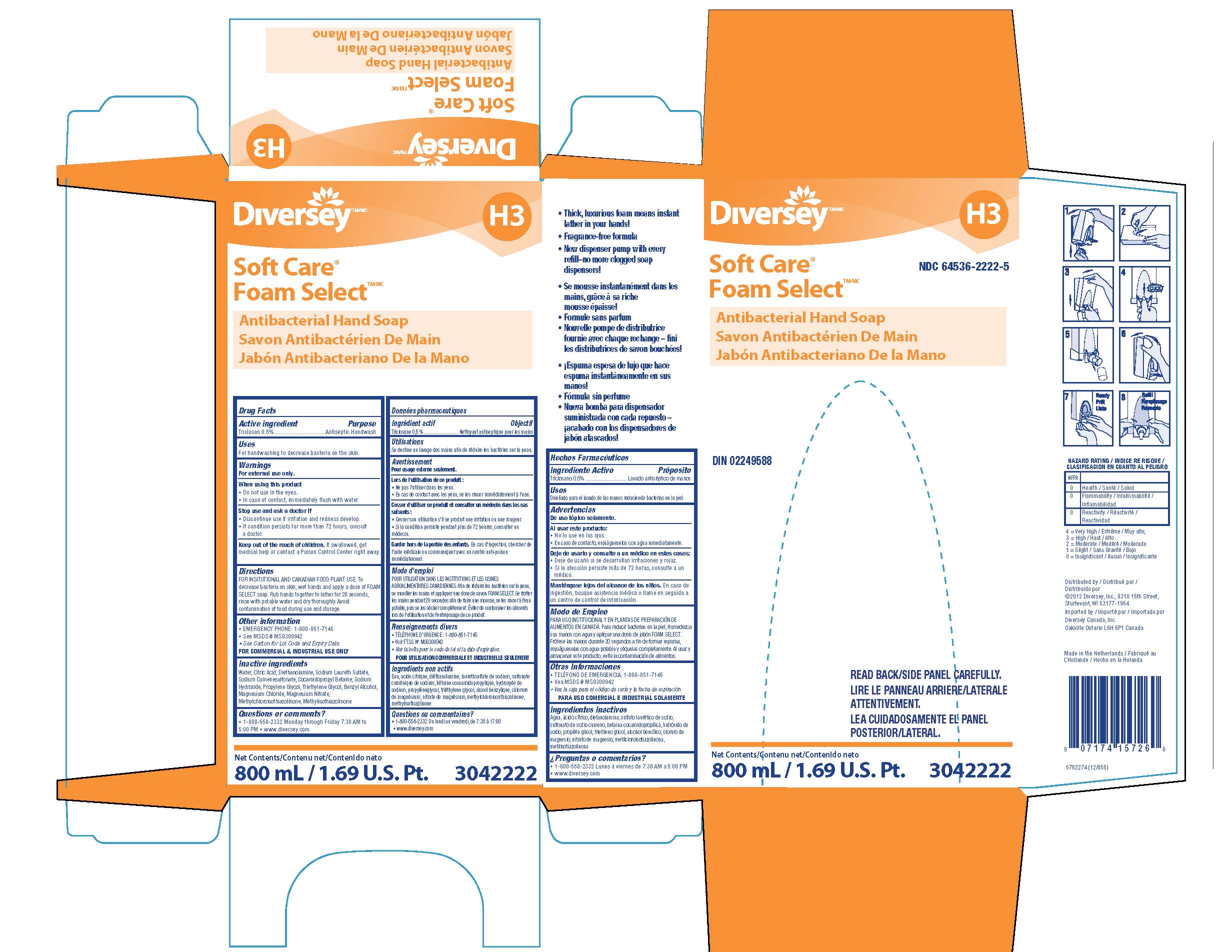
SOFT CARE FOAM SELECT ANTIBACTERIAL HAND- triclosan liquid
Diversey, Inc.
----------
Drug Facts NDC 64536-2222
Warnings
For external use only.
Directions FOR INSTITUTIONAL AND CANADIAN FOOD PLANT USE.
To decrease bacteria on skin, wet hands and apply a dose of FOAM SELECT soap.
Rub hands together to lather for 20 seconds, rinse with potable water and dry thoroughly.
Avoid contamination of food during use and storage.
Other information
EMERGENCY PHONE: 1-800-851-7145
See MSDS MS0300942
See Carton for Lot Code and Expiry Date
FOR COMMERCIAL AND INDUSTRIAL USE ONLY
| SOFT CARE FOAM SELECT ANTIBACTERIAL HAND
triclosan liquid |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Diversey, Inc. (018240817) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Technical Concepts Bentfield b.v. | 489421698 | manufacture(64536-2222) | |
Revised: 12/2023
Document Id: 0c02c592-7161-8490-e063-6294a90a2cb5
Set id: 102a7429-328d-4a6b-9c56-a92bf8bd5ef4
Version: 2
Effective Time: 20231208
Diversey, Inc.