Label: METOCLOPRAMIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-346-15, 21695-346-20, 21695-346-30, 21695-346-60, view more21695-346-90, 21695-485-30, 21695-485-90 - Packager: Rebel Distributors Corp.
- This is a repackaged label.
- Source NDC Code(s): 0591-2228, 0591-2229
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 4, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Metoclopramide hydrochloride is a white or practically white, crystalline, odorless or practically odorless powder. It is very soluble in water, freely soluble in alcohol, sparingly soluble in chloroform, practically insoluble in ether. Chemically, it is 4-amino-5-chloro- N-[2-(diethylamino) ethyl]-2-methoxybenzamide monohydrochloride monohydrate. Its structural formula is as follows:
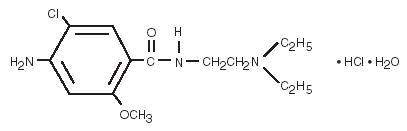
C14H22CIN3O2•HCl•H2O Molecular Weight: 354.3
Each tablet for oral administration contains metoclopramide hydrochloride, equivalent to either 5 mg or 10 mg metoclopramide. Tablets also contain as inactive ingredients anhydrous lactose, magnesium stearate, povidone, pregelatinized starch, sodium starch glycolate and (5 mg only) D&C Yellow #10 and FD&C Blue #1.
-
CLINICAL PHARMACOLOGY
Metoclopramide stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. Its mode of action is unclear. It seems to sensitize tissues to the action of acetylcholine. The effect of metoclopramide on motility is not dependent on intact vagal innervation, but it can be abolished by anticholinergic drugs.
Metoclopramide increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower esophageal sphincter. It has little, if any, effect on the motility of the colon or gallbladder.
In patients with gastroesophageal reflux and low LESP (lower esophageal sphincter pressure), single oral doses of metoclopramide produce dose-related increases in LESP. Effects begin at about 5 mg and increase through 20 mg (the largest dose tested). The increase in LESP from a 5 mg dose lasts about 45 minutes and that of 20 mg lasts between 2 and 3 hours. Increased rate of stomach emptying has been observed with single oral doses of 10 mg.
The antiemetic properties of metoclopramide appear to be a result of its antagonism of central and peripheral dopamine receptors. Dopamine produces nausea and vomiting by stimulation of the medullary chemoreceptor trigger zone (CTZ), and metoclopramide blocks stimulation of the CTZ by agents like l-dopa or apomorphine which are known to increase dopamine levels or to possess dopamine-like effects. Metoclopramide also abolishes the slowing of gastric emptying caused by apomorphine.
Like the phenothiazines and related drugs, which are also dopamine antagonists, metoclopramide produces sedation and may produce extrapyramidal reactions, although these are comparatively rare (see WARNINGS). Metoclopramide inhibits the central and peripheral effects of apomorphine, induces release of prolactin and causes a transient increase in circulating aldosterone levels, which may be associated with transient fluid retention.
The onset of pharmacological action of metoclopramide is 1 to 3 minutes following an intravenous dose, 10 to 15 minutes following intramuscular administration, and 30 to 60 minutes following an oral dose; pharmacological effects persist for 1 to 2 hours.
Pharmacokinetics:
Metoclopramide is rapidly and well absorbed. Relative to an intravenous dose of 20 mg, the absolute oral bioavailability of metoclopramide is 80% ± 15.5% as demonstrated in a crossover study of 18 subjects. Peak plasma concentrations occur at about 1 to 2 hours after a single oral dose. Similar time to peak is observed after individual doses at steady state.
In a single dose study of 12 subjects, the area under the drug concentration-time curve increased linearly with doses from 20 to 100 mg. Peak concentrations increase linearly with dose; time to peak concentrations remains the same; whole body clearance is unchanged; and the elimination rate remains the same. The average elimination halflife in individuals with normal renal function is 5 to 6 hours. Linear kinetic processes adequately describe the absorption and elimination of metoclopramide.
Approximately 85% of the radioactivity of an orally administered dose appears in the urine within 72 hours. Of the 85% eliminated in the urine, about half is present as free or conjugated metoclopramide.
The drug is not extensively bound to plasma proteins (about 30%). The whole body volume of distribution is high (about 3.5 L/kg) which suggests extensive distribution of drug to the tissues.
Renal impairment affects the clearance of metoclopramide. In a study with patients with varying degrees of renal impairment, a reduction in creatinine clearance was correlated with a reduction in plasma clearance, renal clearance, non-renal clearance, and increase in elimination half-life. The kinetics of metoclopramide in the presence of renal impairment remained linear however. The reduction in clearance as a result of renal impairment suggests that adjustment downward of maintenance dosage should be done to avoid drug accumulation.
Adult Pharmacokinetic Data Parameter Value Vd (L/kg) ~ 3.5 Plasma Protein Binding ~ 30% t1/2 (hr) 5-6 Oral Bioavailability 80% ± 15.5% In pediatric patients, the pharmacodynamics of metoclopramide following oral and intravenous administration are highly variable and a concentration-effect relationship has not been established.
There are insufficient reliable data to conclude whether the pharmacokinetics of metoclopramide in adults and the pediatric population are similar. Although there are insufficient data to support the efficacy of metoclopramide in pediatric patients with symptomatic gastroesophageal reflux (GER) its pharmacokinetics have been studied in these patient populations.
In an open-label study, six pediatric patients (age range, 3.5 weeks to 5.4 months) with GER received metoclopramide 0.15 mg/kg oral solution every 6 hours for 10 doses. The mean peak plasma concentration of metoclopramide after the tenth dose was 2-fold (56.8 mcg/L) higher compared to that observed after the first dose (29 mcg/L) indicating drug accumulation with repeated dosing. After the tenth dose, the mean time to reach peak concentrations (2.2 hr), half-life (4.1 hr), clearance (0.67 L/h/kg), and volume of distribution (4.4 L/kg) of metoclopramide were similar to those observed after the first dose. In the youngest patient (age, 3.5 weeks), metoclopramide half-life after the first and the tenth dose (23.1 and 10.3 hr, respectively) was significantly longer compared to other infants due to reduced clearance. This may be attributed to immature hepatic and renal systems at birth.
Pediatric Pharmacokinetic Study Reference Dose, Route t1/2
(hr)Cl
(L/hr/kg)Vd
(L/kg)Cmax
(mcg/L)1. 0.15 mg/kg
oral soln,
multiple dose4.1 a,b 0.67±0.14 4.4±0.65
(Vd area)1st dose = 29±2.3
10th dose = 56.8 ± 10.5a. Data presented as means ± SEM. b. SEM not available. 1. Kearns, GL, et al. J Pediatric Gastroenterol Nutr 7(6):823-829, 1988. -
INDICATIONS AND USAGE
Symptomatic Gastroesophageal Reflux: Metoclopramide tablets are indicated as short-term (4 to 12 weeks) therapy for adults with symptomatic, documented gastroesophageal reflux who fail to respond to conventional therapy.
The principal effect of metoclopramide is on symptoms of postprandial and daytime heartburn with less observed effect on nocturnal symptoms. If symptoms are confined to particular situations, such as following the evening meal, use of metoclopramide as single doses prior to the provocative situation should be considered, rather than using the drug throughout the day. Healing of esophageal ulcers and erosions has been endoscopically demonstrated at the end of a 12-week trial using doses of 15 mg q.i.d. As there is no documented correlation between symptoms and healing of esophageal lesions, patients with documented lesions should be monitored endoscopically.
Diabetic Gastroparesis (Diabetic Gastric Stasis): Metoclopramide is indicated for the relief of symptoms associated with acute and recurrent diabetic gastric stasis. The usual manifestations of delayed gastric emptying (e.g., nausea, vomiting, heartburn, persistent fullness after meals, and anorexia) appear to respond to metoclopramide within different time intervals. Significant relief of nausea occurs early and continues to improve over a three week period. Relief of vomiting and anorexia may precede the relief of abdominal fullness by one week or more.
-
CONTRAINDICATIONS
Metoclopramide should not be used whenever stimulation of gastrointestinal motility might be dangerous, e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation.
Metoclopramide is contraindicated in patients with pheochromocytoma because the drug may cause a hypertensive crisis, probably due to release of catecholamines from the tumor. Such hypertensive crises may be controlled by phentolamine.
Metoclopramide is contraindicated in patients with known sensitivity or intolerance to the drug.
Metoclopramide should not be used in epileptics or patients receiving other drugs which are likely to cause extrapyramidal reactions, since the frequency and severity of seizures or extrapyramidal reactions may be increased.
-
WARNINGS
Mental depression has occurred in patients with and without prior history of depression. Symptoms have ranged from mild to severe and have included suicidal ideation and suicide. Metoclopramide should be given to patients with a prior history of depression only if the expected benefits outweigh the potential risks.
Extrapyramidal symptoms, manifested primarily as acute dystonic reactions, occur in approximately 1 in 500 patients treated with the usual adult dosages of 30 to 40 mg/day of metoclopramide. These usually are seen during the first 24 to 48 hours of treatment with metoclopramide, occur more frequently in pediatric patients and adult patients less than 30 years of age and are even more frequent at the higher doses. These symptoms may include involuntary movements of limbs and facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of tongue, bulbar type of speech, trismus, or dystonic reactions resembling tetanus. Rarely, dystonic reactions may present as stridor and dyspnea, possibly due to laryngo-spasm. If these symptoms should occur, inject 50 mg of Benadryl® (diphenhydramine hydrochloride) intramuscularly, and they usually will subside. Cogentin® (benztropine mesylate), 1 to 2 mg intramuscularly, may also be used to reverse these reactions.
Parkinsonian-like symptoms have occurred, more commonly within the first 6 months after beginning treatment with metoclopramide, but occasionally after longer periods. These symptoms generally subside within 2 to 3 months following discontinuance of metoclopramide. Patients with preexisting Parkinson’s disease should be given metoclopramide cautiously, if at all, since such patients may experience exacerbation of parkinsonian symptoms when taking metoclopramide.
Tardive Dyskinesia: Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with metoclopramide. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients are likely to develop the syndrome.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase with the duration of treatment and the total cumulative dose.
Less commonly, the syndrome can develop after relatively brief treatment periods at low doses; in these cases, symptoms appear more likely to be reversible.
There is no known treatment for established cases of tardive dyskinesia although the syndrome may remit, partially or completely, within several weeks-to-months after metoclopramide is withdrawn. Metoclopramide itself, however, may suppress (or partially suppress) the signs of tardive dyskinesia, thereby masking the underlying disease process. The effect of this symptomatic suppression upon the long-term course of the syndrome is unknown. Therefore, the use of metoclopramide for the symptomatic control of tardive dyskinesia is not recommended.
-
PRECAUTIONS
General:
In one study in hypertensive patients, intravenously administered metoclopramide was shown to release catecholamines; hence, caution should be exercised when metoclopramide is used in patients with hypertension.
Because metoclopramide produces a transient increase in plasma aldosterone, certain patients, especially those with cirrhosis or congestive heart failure, may be at risk of developing fluid retention and volume overload. If these side effects occur at any time during metoclopramide therapy, the drug should be discontinued.
Giving a promotility drug such as metoclopramide theoretically could put increased pressure on suture lines following a gut anastomosis or closure. This possibility should be considered and weighed when deciding whether to use metoclopramide or nasogastric suction in the prevention of postoperative nausea and vomiting.
Information for Patients:
Metoclopramide may impair the mental and/or physical abilities required for the performance of hazardous tasks such as operating machinery or driving a motor vehicle. The ambulatory patient should be cautioned accordingly.
Drug Interactions:
The effects of metoclopramide on gastrointestinal motility are antagonized by anticholinergic drugs and narcotic analgesics. Additive sedative effects can occur when metoclopramide is given with alcohol, sedatives, hypnotics, narcotics or tranquilizers.
The finding that metoclopramide releases catecholamines in patients with essential hypertension suggests that it should be used cautiously, if at all, in patients receiving monoamine oxidase inhibitors.
Absorption of drugs from the stomach may be diminished (e.g., digoxin) by metoclopramide, whereas the rate and/or extent of absorption of drugs from the small bowel may be increased (e.g., acetaminophen, tetracycline, levodopa, ethanol, cyclosporine).
Gastroparesis (gastric stasis) may be responsible for poor diabetic control in some patients. Exogenously administered insulin may begin to act before food has left the stomach and lead to hypoglycemia. Because the action of metoclopramide will influence the delivery of food to the intestines and thus the rate of absorption, insulin dosage or timing of dosage may require adjustment.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
A 77-week study was conducted in rats with oral doses up to about 40 times the maximum recommended human daily dose. Metoclopramide elevates prolactin levels and the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of metoclopramide is contemplated in a patient with previously detected breast cancer. Although disturbances, such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported with prolactin-elevating drugs, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of prolactin-stimulating neuroleptic drugs and metoclopramide. Neither clinical studies nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is too limited to be conclusive at this time.
An Ames mutagenicity test performed on metoclopramide was negative.
Pregnancy Category B:
Reproduction studies performed in rats, mice, and rabbits by the I.V., I.M., S.C., and oral routes at maximum levels ranging from 12 to 250 times the human dose have demonstrated no impairment of fertility or significant harm to the fetus due to metoclopramide. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers:
Metoclopramide is excreted in human milk. Caution should be exercised when metoclopramide is administered to a nursing mother.
Pediatric Use:
Safety and effectiveness in pediatric patients have not been established except as stated to facilitate small bowel intubation (see OVERDOSAGE and DOSAGE AND ADMINISTRATION).
Care should be exercised in administering metoclopramide to neonates since prolonged clearance may produce excessive serum concentrations (see CLINICAL PHARMACOLOGY—Pharmacokinetics). In addition, neonates have reduced levels of nicotinamide adenine dinucleotide-methemoglobin reductase which, in combination with the aforementioned pharmacokinetic factors, make neonates more susceptible to methemoglobinemia (see OVERDOSAGE).
The safety profile of metoclopramide in adults cannot be extrapolated to pediatric patients. Dystonias and other extrapyramidal reactions associated with metoclopramide are more common in the pediatric population than in adults. (See WARNINGS and ADVERSE REACTIONS—Extrapyramidal Reactions.)
-
ADVERSE REACTIONS
In general, the incidence of adverse reactions correlates with the dose and duration of metoclopramide administration. The following reactions have been reported, although in most instances, data do not permit an estimate of frequency.
CNS Effects: Restlessness, drowsiness, fatigue, and lassitude occur in approximately 10% of patients receiving the most commonly prescribed dosage of 10 mg q.i.d. (see PRECAUTIONS). Insomnia, headache, confusion, dizziness, or mental depression with suicidal ideation (see WARNINGS) occur less frequently. There are isolated reports of convulsive seizures without clearcut relationships to metoclopramide. Rarely, hallucinations have been reported.
Extrapyramidal Reactions (EPS): Acute dystonic reactions, the most common type of EPS associated with metoclopramide, occur in approximately 0.2% of patients (1 in 500) treated with 30 to 40 mg of metoclopramide per day. Symptoms include involuntary movements of limbs, facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of tongue, bulbar type of speech, trismus, opisthotonus (tetanus-like reactions), and rarely, stridor and dyspnea possibly due to laryngospasm; ordinarily these symptoms are readily reversed by diphenhydramine (see WARNINGS).
Parkinsonian-like symptoms may include bradykinesia, tremor, cogwheel rigidity, masklike facies (see WARNINGS).
Tardive dyskinesia most frequently is characterized by involuntary movements of the tongue, face, mouth, or jaw, and sometimes by involuntary movements of the trunk and/or extremities; movements may be choreoathetotic in appearance (see WARNINGS).
Motor restlessness (akathisia) may consist of feelings of anxiety, agitation, jitteriness, and insomnia, as well as inability to sit still, pacing, foot-tapping. These symptoms may disappear spontaneously or respond to a reduction in dosage.
Endocrine Disturbances: Galactorrhea, amenorrhea, gynecomastia, impotence secondary to hyperprolactinemia (see PRECAUTIONS). Fluid retention secondary to transient elevation of aldosterone (see CLINICAL PHARMACOLOGY).
Cardiovascular: Hypotension, hypertension, supraventricular tachycardia, bradycardia, fluid retention, acute congestive heart failure and possible AV block (see CONTRAINDICATIONS and PRECAUTIONS).
Gastrointestinal: Nausea and bowel disturbances, primarily diarrhea.
Hepatic: Rarely, cases of hepatotoxicity, characterized by such findings as jaundice and altered liver function tests, when metoclopramide was administered with other drugs with known hepatotoxic potential.
Renal: Urinary frequency and incontinence.
Hematologic: A few cases of neutropenia, leukopenia, or agranulocytosis, generally without clearcut relationships to metoclopramide. Methemoglobinemia, especially with overdosage in neonates (see OVERDOSAGE).
Sulfhemoglobinemia in adults.
Allergic Reactions: A few cases of rash, urticaria, or bronchospasm, especially in patients with a history of asthma. Rarely, angioneurotic edema, including glossal or laryngeal edema.
Miscellaneous: Visual disturbances. Porphyria. Rare occurrences of neuroleptic malignant syndrome (NMS) have been reported. This potentially fatal syndrome is comprised of the symptom complex of hyperthermia, altered consciousness, muscular rigidity, and autonomic dysfunction.
Transient flushing of the face and upper body, without alterations in vital signs, following high doses intravenously.
-
OVERDOSAGE
Symptoms of overdosage may include drowsiness, disorientation and extrapyramidal reactions. Anticholinergic or antiparkinson drugs or antihistamines with anticholinergic properties may be helpful in controlling the extrapyramidal reactions. Symptoms are self-limiting and usually disappear within 24 hours.
Hemodialysis removes relatively little metoclopramide, probably because of the small amount of the drug in blood relative to tissues. Similarly, continuous ambulatory peritoneal dialysis does not remove significant amounts of drug. It is unlikely that dosage would need to be adjusted to compensate for losses through dialysis. Dialysis is not likely to be an effective method of drug removal in overdose situations.
Unintentional overdose due to misadministration has been reported in infants and children with the use of metoclopramide oral solution. While there was no consistent pattern to the reports associated with these overdoses, events included seizures, extrapyramidal reactions, and lethargy.
Methemoglobinemia has occurred in premature and full-term neonates who were given overdoses of metoclopramide (1 to 4 mg/kg/day orally, intramuscularly or intravenously for 1 to 3 or more days).
Methemoglobinemia has not been reported in neonates treated with 0.5 mg/kg/day in divided doses. Methemoglobinemia can be reversed by the intravenous administration of methylene blue.
-
DOSAGE AND ADMINISTRATION
For the Relief of Symptomatic Gastroesophageal Reflux: Administer from 10 mg to 15 mg metoclopramide orally up to q.i.d. 30 minutes before each meal and at bedtime, depending upon symptoms being treated and clinical response (see CLINICAL PHARMACOLOGY and INDICATIONS AND USAGE). If symptoms occur only intermittently or at specific times of the day, use of metoclopramide in single doses up to 20 mg prior to the provoking situation may be preferred rather than continuous treatment. Occasionally, patients (such as elderly patients) who are more sensitive to the therapeutic or adverse effects of metoclopramide will require only 5 mg per dose.
Experience with esophageal erosions and ulcerations is limited, but healing has thus far been documented in one controlled trial using q.i.d. therapy at 15 mg per dose, and this regimen should be used when lesions are present, so long as it is tolerated (see ADVERSE REACTIONS). Because of the poor correlation between symptoms and endoscopic appearance of the esophagus, therapy directed at esophageal lesions is best guided by endoscopic evaluation.
Therapy longer than 12 weeks has not been evaluated and cannot be recommended. For the Relief of Symptoms Associated with Diabetic Gastroparesis (Diabetic Gastric Stasis): Administer 10 mg of metoclopramide 30 minutes before each meal and at bedtime for two to eight weeks, depending upon response and the likelihood of continued well-being upon drug discontinuation.
The initial route of administration should be determined by the severity of the presenting symptoms. If only the earliest manifestations of diabetic gastric stasis are present, oral administration of metoclopramide may be initiated. However, if severe symptoms are present, therapy should begin with Metoclopramide Injection (I.M. or I.V.) (consult labeling of the injection prior to initiating parenteral administration).
Administration of the Metoclopramide Injection up to 10 days may be required before symptoms subside, at which time oral administration may be instituted. Since diabetic gastric stasis is frequently recurrent, metoclopramide therapy should be reinstituted at the earliest manifestation.
Use in Patients with Renal or Hepatic Impairment: Since metoclopramide is excreted principally through the kidneys, in those patients whose creatinine clearance is below 40 mL/min, therapy should be initiated at approximately one-half the recommended dosage. Depending upon clinical efficacy and safety considerations, the dosage may be increased or decreased as appropriate.
See OVERDOSAGE section for information regarding dialysis.
Metoclopramide undergoes minimal hepatic metabolism, except for simple conjugation. Its safe use has been described in patients with advanced liver disease whose renal function was normal.
-
HOW SUPPLIED
Metoclopramide Tablets, USP:
5 mg - Light green, round, unscored tablets in bottles of 30 and 90. Debossed: PLIVA 517
10 mg - White, round, scored tablets in bottles of 15, 20, 30, 60 and 90. Debossed: PLIVA 430
Dispense in a tight, light-resistant container as defined in the USP.
Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature].
Manufactured by:
BARR LABORATORIES, INC.
Pomona, NY 10970 USADistributed by:
Watson Pharma, Inc.
Corona, CA 92880 USARepackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METOCLOPRAMIDE
metoclopramide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-485(NDC:0591-2228) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOCLOPRAMIDE HYDROCHLORIDE (UNII: W1792A2RVD) (METOCLOPRAMIDE - UNII:L4YEB44I46) METOCLOPRAMIDE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color GREEN (light green) Score no score Shape ROUND Size 6mm Flavor Imprint Code PLIVA;517 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-485-30 30 in 1 BOTTLE 2 NDC:21695-485-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA072750 01/01/2008 METOCLOPRAMIDE
metoclopramide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-346(NDC:0591-2229) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOCLOPRAMIDE HYDROCHLORIDE (UNII: W1792A2RVD) (METOCLOPRAMIDE - UNII:L4YEB44I46) METOCLOPRAMIDE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code PLIVA;430 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-346-15 15 in 1 BOTTLE 2 NDC:21695-346-20 20 in 1 BOTTLE 3 NDC:21695-346-30 30 in 1 BOTTLE 4 NDC:21695-346-60 60 in 1 BOTTLE 5 NDC:21695-346-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA071250 01/01/2006 Labeler - Rebel Distributors Corp. (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp. 118802834 RELABEL, REPACK


