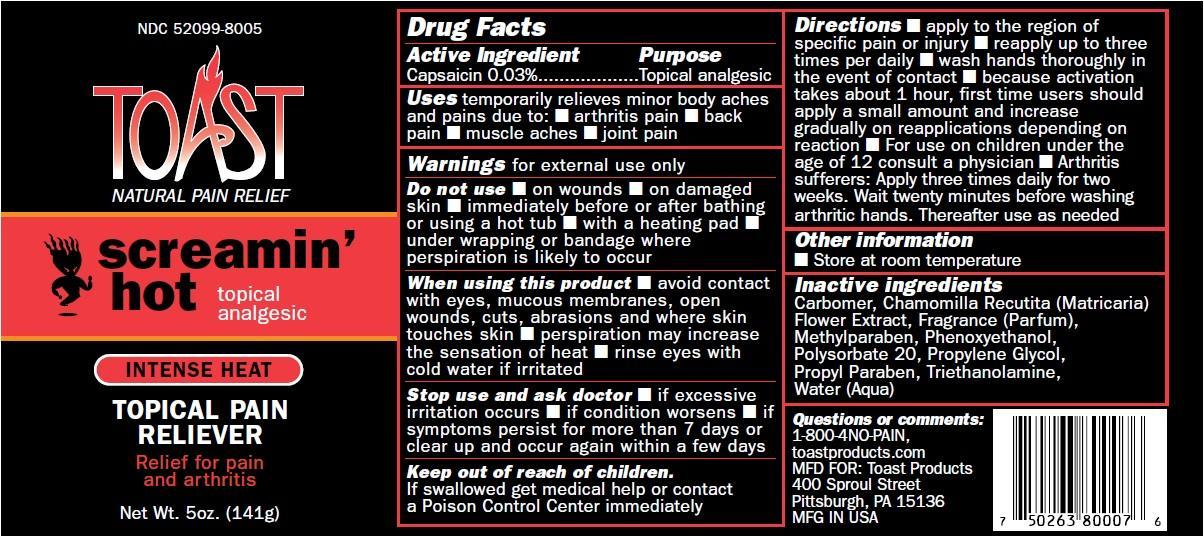
Label: SCREAMIN HOT TOAST- capsaicin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 52099-8005-1 - Packager: Q.A. Laboratories
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Directions apply to the region of specific pain or injury, reapply up to three times per daily, wash hands thoroughly in the event of contact, because activation takes about 1 hour, first time users should apply a small amount and increase gradually on reapplications depending on reaction.For use on children under the age of 12 consult a physician. Arthritis sufferers: Apply three times daily for two weeks. Wait twenty minutes before washing arthritic hands. Thereafter use as needed.
- SAFE HANDLING WARNING
- INACTIVE INGREDIENT
- QUESTIONS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SCREAMIN HOT TOAST
capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52099-8005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 3 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CHAMOMILE (UNII: FGL3685T2X) DMDM HYDANTOIN (UNII: BYR0546TOW) IMIDUREA (UNII: M629807ATL) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52099-8005-1 141 g in 1 TUBE; Type 0: Not a Combination Product 06/19/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/19/2012 Labeler - Q.A. Laboratories (065361149) Registrant - Q.A. Laboratories (065361149) Establishment Name Address ID/FEI Business Operations Q.A. Laboratories 065361149 manufacture(52099-8005)