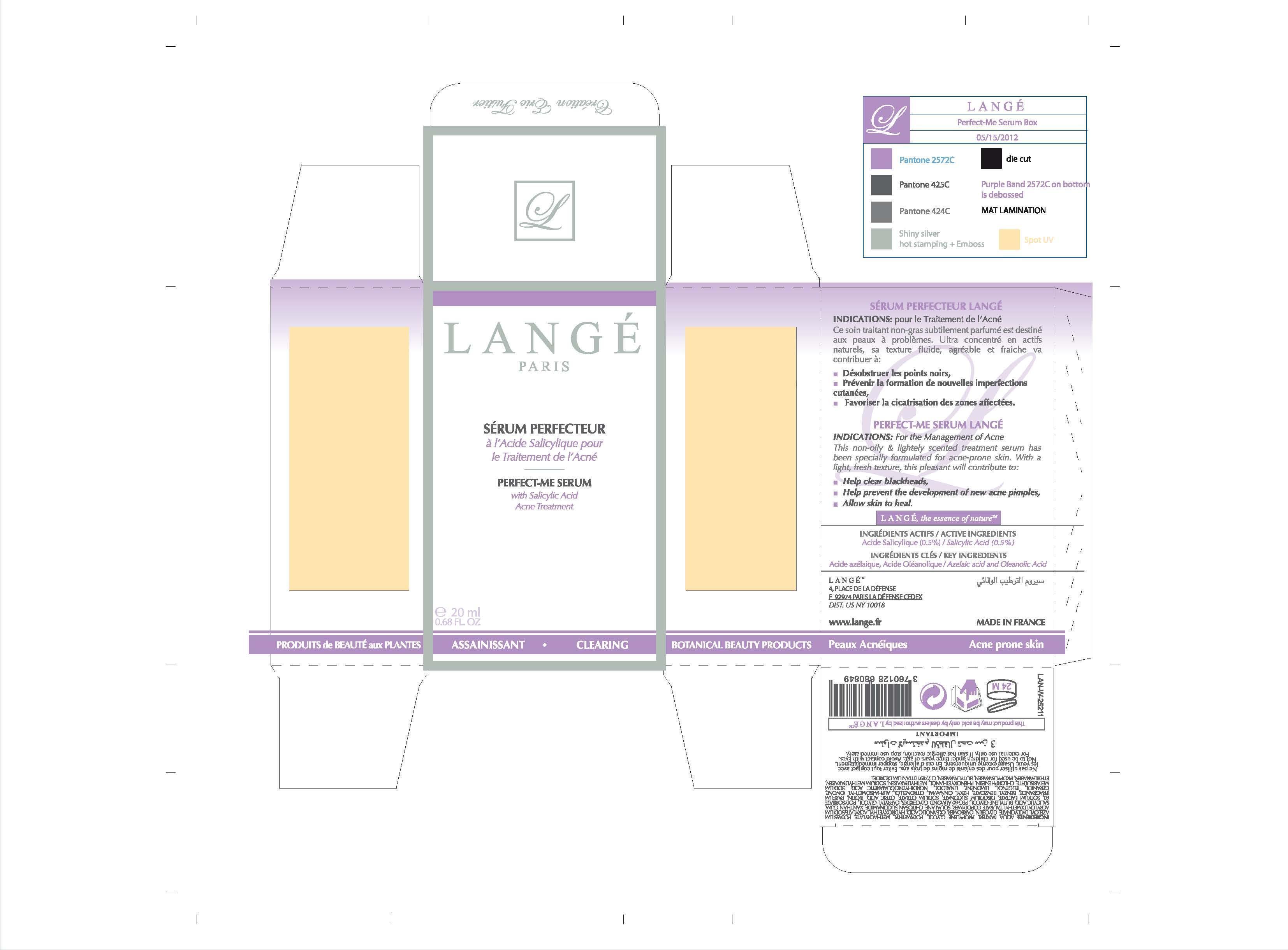
Label: PERFECT ME SERUM- salicylic acid cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 51830-052-08 - Packager: Lange SAS
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 12, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
PERFECT-ME SERUM LANGE
INDICATIONS: For the Management of Acne
This non-oily and lightely scented treatment serum has been specially formulated for acne-prone skin. With a light, fresh texture, this pleasant will contribute to:
Help clear blackheads,
Help prevent the development of new acne pimples,
Allow skin to heal. - WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INACTIVE INGREDIENT
WATER
PROPYLENE GLYCOL
POLYMETHYL METHACRYLATE
POTASSIUM AZELOYL DIGLYCINATE
GLYCERIN
CARBOMER
OLEANOLIC ACID
HYDROXYETHYL ACRYLATE/SODIUM
ACRYLOYLDIMETHYLTAURATE
COPOLYMER
SQUALANE
CHITOSAN SUCCINAMIDE
XANTHAN GUM
SALICYLIC ACID
BUTYLENE GLYCOL
PEG-60 ALMOND GLYCERIDES
CAPRYLYL GLYCOL
POLYSORBATE 60
SODIUM LACTATE
DISODIUM SUCCINATE
SODIUM CITRATE
CITRIC ACID
BIOTIN
BENZYL BENZOATE
HEXYL CINNAMAL
CITRONELLOL
ALPHA-ISOMETHYL IONONE
GERANIOL
EUGENOL
LIMONENE
LINALOOL
NORDIHYDROGUAIARETIC ACID
SODIUM METABISULFITE
CHLORPHENESIN
PHENOXYETHANOL
METHYLPARABEN
SODIUM METHYLPARABEN
BUTYLPARABEN
ETHYLPARABEN
PROPYLPARABEN
ISOBUTYLPARABEN
CI 77891 (TITANIUM DIOXIDE) -
INGREDIENTS AND APPEARANCE
PERFECT ME SERUM
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51830-052 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1 mg in 20 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POTASSIUM AZELOYL DIGLYCINATE (UNII: N02RVN6NYP) GLYCERIN (UNII: PDC6A3C0OX) OLEANOLIC ACID (UNII: 6SMK8R7TGJ) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) SQUALANE (UNII: GW89575KF9) XANTHAN GUM (UNII: TTV12P4NEE) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PEG-60 ALMOND GLYCERIDES (UNII: 4Y0E651N0F) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POLYSORBATE 60 (UNII: CAL22UVI4M) SODIUM LACTATE (UNII: TU7HW0W0QT) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) SODIUM CITRATE (UNII: 1Q73Q2JULR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BIOTIN (UNII: 6SO6U10H04) BENZYL BENZOATE (UNII: N863NB338G) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) GERANIOL (UNII: L837108USY) EUGENOL (UNII: 3T8H1794QW) LINALOOL, (+)- (UNII: F4VNO44C09) MASOPROCOL (UNII: 7BO8G1BYQU) SODIUM METABISULFITE (UNII: 4VON5FNS3C) CHLORPHENESIN (UNII: I670DAL4SZ) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) METHYLPARABEN SODIUM (UNII: CR6K9C2NHK) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51830-052-08 20 mg in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/18/2012 Labeler - Lange SAS (275956105) Establishment Name Address ID/FEI Business Operations Lange SAS 275956105 manufacture(51830-052)