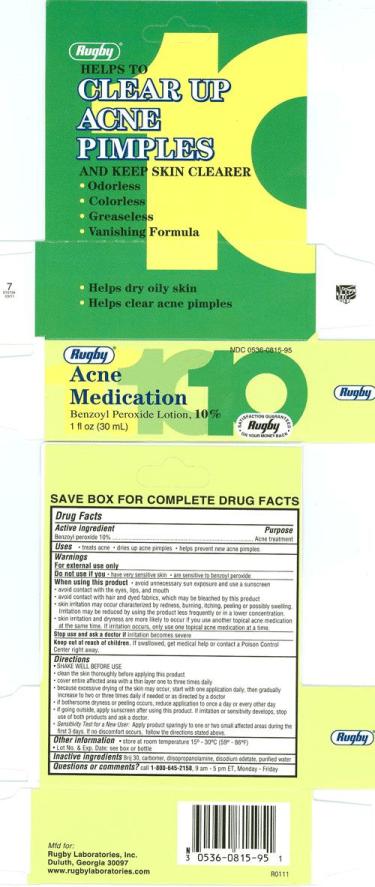
ACNE MEDICATION 10- benzoyl peroxide lotion
Rugby Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Keep Out of Reach of Children
Keep out of reach of children.
If swallowed, get medical help or contact Poison Control Center right away.
Warnings
For external use only
Do not use:
if you have very sensitive skin or if you are sensitive to benzoyl peroxide.
Directions
shake well
clean the skin thoroughly before applying this product
cover entire affected area with a thin layer one to three times daily
because excessive drying of the skin may occur, start with one application daily, then gradual increase to two three times daily or as directed by a doctor
if bothersome dryness or peeling occurs, reduce application to once a day or every other day
if going outside use sunscreen after using this product.
if irritation or sensitivity develops, discontinue use of both products and consult a doctor.
Sensitivity Test for New Users: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occrs, follow the directions stated above
Other Safety Information
Other Information
to prevent bleaching, avoid contact with hair or dyed fabric and clothing
KEEP TIGHTLY CLOSE
store at room temperature 15°-30°C (59°-86°F)
Lot No. & Exp. Date: See box or bottle
Distributed by:
Rugby Laboratories
Livonia, MI 48150 USA
Questions?
Call 1-(800) 645-2158
| ACNE MEDICATION 10
benzoyl peroxide lotion |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |