Label: EXPAREL- bupivacaine injection, suspension, liposomal
-
NDC Code(s):
65250-133-04,
65250-133-09,
65250-133-10,
65250-266-04, view more65250-266-09, 65250-266-20
- Packager: Pacira Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 30, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EXPAREL® safely and effectively. See full prescribing information for EXPAREL.
EXPAREL (bupivacaine liposome injectable suspension), for infiltration or perineural use
Initial U.S. Approval: 1972INDICATIONS AND USAGE
EXPAREL contains bupivacaine, an amide local anesthetic, and is indicated to produce postsurgical:
- Local analgesia via infiltration in patients aged 6 years and older (1).
- Regional analgesia via an interscalene brachial plexus nerve block in adults (1).
- Regional analgesia via a sciatic nerve block in the popliteal fossa in adults (1).
- Regional analgesia via an adductor canal block in adults (1).
Limitations of Use
The safety and effectiveness of EXPAREL have not been established to produce postsurgical regional analgesia via other nerve blocks besides an interscalene brachial plexus nerve block, a sciatic nerve block in the popliteal fossa, or an adductor canal block.
DOSAGE AND ADMINISTRATION
- EXPAREL is for single administration only (2.1).
- EXPAREL is not substitutable with other bupivacaine products even if the strength is the same. Therefore, it is not possible to convert a dose from other bupivacaine products to an EXPAREL dose and vice versa (2.1, 2.5).
- Do not dilute EXPAREL with water or other hypotonic solutions (2.1).
- The recommended dose of EXPAREL for:
- Local infiltration in adults is up to a maximum dose of 266 mg. See Full Prescribing Information for guidance on dose selection (2.2).
- Local infiltration in pediatric patients aged 6 to less than 17 years is 4 mg/kg, up to a maximum of 266 mg (2.2).
- Interscalene brachial plexus nerve block in adults is 133 mg (2.3).
- Sciatic nerve block in the popliteal fossa in adults is 133 mg (2.3).
- Adductor canal block in adults is 133 mg (10 mL) admixed with 50 mg (10 mL) 0.5% bupivacaine HCl, for a total volume of 20 mL (2.3).
- For all these nerve blocks, administer additional analgesics, which may include other immediate-release local anesthetics, as appropriate (2.3).
- See Full Prescribing Information for important preparation and administration instructions and compatibility considerations (2.4, 2.5).
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
EXPAREL is contraindicated in obstetrical paracervical block anesthesia (4).
WARNINGS AND PRECAUTIONS
- Monitor cardiovascular status, neurological status, and vital signs during and after injection of EXPAREL (5.1).
- Because amide-type local anesthetics, such as bupivacaine, are metabolized by the liver, use EXPAREL cautiously in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations (5.1).
- Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use (5.1).
- Avoid additional use of local anesthetics within 96 hours following administration of EXPAREL (5.2).
ADVERSE REACTIONS
Adverse reactions reported with an incidence greater than or equal to 10% following EXPAREL administration via:
- Infiltration in adults were nausea, constipation, and vomiting (6.1).
- Nerve block in adults were nausea, pyrexia, headache, and constipation (6.1).
- Infiltration in pediatric patients six to less than 17 years of age were nausea, vomiting, constipation, hypotension, anemia, muscle twitching, blurred vision, pruritus, and tachycardia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Pacira Pharmaceuticals, Inc. at 1-855-RX-EXPAREL (1-855-793-9727) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Lidocaine or other non-bupivacaine local anesthetics: Do not admix with EXPAREL. EXPAREL may be administered at least 20 minutes or more following local administration of lidocaine (7).
- Bupivacaine HCl: Do not exceed a milligram dose of bupivacaine HCl solution to EXPAREL of 1:2 when admixing, as this may impact the pharmacokinetics and/or physicochemical properties of the drugs (7).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dose, Preparation, and Administration Instructions
2.2 Recommended Dose for Local Analgesia via Infiltration
2.3 Recommended Dose for Regional Analgesia
2.4 Preparation and Administration Instructions
2.5 Compatibility Considerations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Warnings and Precautions for Bupivacaine-Containing Products
5.2 Warnings and Precautions Specific for EXPAREL
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies in Adult Patients
14.2 Studies in Adults to Produce Postsurgical Local Analgesia
14.3 Nerve Block Studies in Adults to Produce Postsurgical Regional Analgesia
14.4 Studies That Do Not Support an Indication for Certain Nerve Blocks in Adults
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
EXPAREL is indicated to produce postsurgical:
- Local analgesia via infiltration in patients aged 6 years and older
- Regional analgesia via an interscalene brachial plexus nerve block in adults
- Regional analgesia via a sciatic nerve block in the popliteal fossa in adults
- Regional analgesia via an adductor canal block in adults
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dose, Preparation, and Administration Instructions
- EXPAREL is for single administration only.
- EXPAREL is not substitutable with other bupivacaine products even if the strength is the same. Therefore, it is not possible to convert a dose from other bupivacaine products to an EXPAREL dose and vice versa.
- Do not dilute EXPAREL with water or other hypotonic agents, as it will result in disruption of the liposomal particles.
- Do not administer EXPAREL if it is suspected that the vial has been frozen or exposed to high temperature (greater than 40°C or 104°F) for an extended period.
- Inspect EXPAREL visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer EXPAREL if the product is discolored.
- Do not heat or autoclave before use.
- Do not filter during administration.
2.2 Recommended Dose for Local Analgesia via Infiltration
Local Analgesia via Infiltration in Adults
The recommended dose of EXPAREL for local infiltration in adults is up to a maximum dose of 266 mg, and is based on the following factors:
- Size of the surgical site
- Volume required to cover the area
- Individual patient factors that may impact the safety of an amide local anesthetic
As general guidance in selecting the proper EXPAREL dose for local infiltration in adults, two examples are provided [see Clinical Studies (14.2)]. In adult patients undergoing:
- Bunionectomy, a total of 106 mg (8 mL) of EXPAREL was administered, with 7 mL infiltrated into the tissues surrounding the osteotomy, and 1 mL infiltrated into the subcutaneous tissue.
- Hemorrhoidectomy, a total of 266 mg (20 mL) of EXPAREL was diluted with 10 mL of saline, for a total of 30 mL, divided into six 5 mL aliquots, injected by visualizing the anal sphincter as a clock face and slowly infiltrating one aliquot to each of the even numbers to produce a field block.
Local Analgesia via Infiltration in Pediatric Patients
The recommended dose of EXPAREL for one-time infiltration in pediatric patients, aged 6 to less than 17 years, is 4 mg/kg (up to a maximum of 266 mg), and is based upon two studies of pediatric patients undergoing either spine surgery or cardiac surgery [see Use in Specific Populations (8.4)].
2.3 Recommended Dose for Regional Analgesia
The maximum recommended dose of EXPAREL via perineural use for interscalene brachial plexus nerve block, sciatic nerve block in the popliteal fossa, and adductor canal block is 133 mg. For all these nerve blocks, administer additional analgesics, which may include other immediate-release local anesthetics, as appropriate (for example, Mayo field block for bunionectomy, infiltration between the popliteal artery and capsule of the knee (IPACK) block for total knee arthroplasty).
Regional Analgesia via Interscalene Brachial Plexus Nerve Block in Adults
The recommended dose of EXPAREL for interscalene brachial plexus nerve block in adults is 133 mg and is based upon one study of patients undergoing either total shoulder arthroplasty or rotator cuff repair [see Clinical Studies (14.3)].
Regional Analgesia via Sciatic Nerve Block in the Popliteal Fossa in Adults
The recommended dose of EXPAREL for sciatic nerve block in the popliteal fossa in adults is 133 mg and is based upon one study of patients undergoing bunionectomy [see Clinical Studies (14.3)].
Regional Analgesia via Adductor Canal Block in Adults
The recommended dose of EXPAREL for adductor canal block in adults is 133 mg (10 mL) admixed with 50 mg (10 mL) 0.5% bupivacaine HCl, for a total volume of 20 mL, and is based upon one study of patients undergoing total knee arthroplasty [see Clinical Studies (14.3)].
2.4 Preparation and Administration Instructions
- Invert vials of EXPAREL multiple times to re-suspend the particles immediately prior to withdrawal from the vial.
- Administer EXPAREL (1) undiluted or (2) diluted to increase volume up to a final concentration of 0.89 mg/mL (i.e., 1:14 dilution by volume) with 0.9% preservative-free Sodium Chloride Injection or lactated Ringer's solution. Use diluted EXPAREL within 4 hours of preparation in a syringe.
- Administer EXPAREL with a 25 gauge or larger bore needle to maintain the structural integrity of the liposomal bupivacaine particles.
- Administer EXPAREL slowly via infiltration or perineural use with frequent aspiration to check for blood and minimize the risk of inadvertent intravascular injection.
- Discard unused portion.
2.5 Compatibility Considerations
Some physicochemical incompatibilities exist between EXPAREL and certain other drugs. Direct contact of EXPAREL with these drugs results in a rapid increase in free (unencapsulated) bupivacaine, altering EXPAREL characteristics and potentially affecting the safety and efficacy of EXPAREL. Therefore, admixing EXPAREL with other drugs prior to administration is not recommended [see Drug Interactions (7)].
- Non-bupivacaine based local anesthetics, including lidocaine, may cause an immediate release of bupivacaine from EXPAREL if administered together locally. The administration of EXPAREL may follow the administration of lidocaine after a delay of 20 minutes or more.
- Bupivacaine HCl administered together with EXPAREL may impact the pharmacokinetic and/or physicochemical properties of EXPAREL, and this effect is concentration dependent. Therefore, bupivacaine HCl and EXPAREL may be administered simultaneously in the same syringe, and bupivacaine HCl may be injected immediately before EXPAREL if the ratio of the milligram dose of bupivacaine HCl solution to EXPAREL does not exceed 1:2.
The toxic effects of these drugs are additive, and their administration should be used with caution including monitoring for neurologic and cardiovascular effects related to local anesthetic systemic toxicity [see Warnings and Precautions (5.1) and Overdosage (10)]. - When a topical antiseptic such as povidone iodine (e.g., Betadine) is applied, the site should be allowed to dry before EXPAREL is administered into the site. EXPAREL should not be allowed to come into contact with antiseptics such as povidone iodine in solution.
Studies conducted with EXPAREL demonstrated that the most common implantable materials (polypropylene, PTFE, silicone, stainless steel, and titanium) are not affected by the presence of EXPAREL any more than they are by saline. None of the materials studied had an adverse effect on EXPAREL.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
EXPAREL is contraindicated in obstetrical paracervical block anesthesia [see Use in Specific Populations (8.1)]. While EXPAREL has not been tested with this technique, the use of bupivacaine HCl with this technique has resulted in fetal bradycardia and death.
-
5 WARNINGS AND PRECAUTIONS
5.1 Warnings and Precautions for Bupivacaine-Containing Products
The safety and effectiveness of EXPAREL, other bupivacaine products, and other amide-containing products depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. As there is a potential risk of severe life-threatening adverse reactions associated with the administration of bupivacaine, any bupivacaine-containing product should be administered in a setting where trained personnel and equipment are available to promptly treat patients who show evidence of neurological or cardiac toxicity [see Overdosage (10)].
Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient's state of consciousness should be performed after injection of bupivacaine and other amide-containing products. Restlessness, anxiety, incoherent speech, lightheadedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, depression, or drowsiness may be early warning signs of central nervous system toxicity.
EXPAREL, other bupivacaine products, and other amide-containing products should also be used with caution in patients with impaired cardiovascular function because they may be less able to compensate for functional changes associated with the prolongation of AV conduction produced by these drugs.
Injection of multiple doses of EXPAREL, other bupivacaine products, and other amide-containing products may cause significant increases in plasma concentrations with each repeated dose due to slow accumulation of the drug or its metabolites, or to slow metabolic degradation. Tolerance to elevated blood concentrations varies with the status of the patient.
Because amide-type local anesthetics, such as bupivacaine, are metabolized by the liver, these drugs should be used cautiously in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations.
Central Nervous System Reactions
The incidences of adverse neurologic reactions associated with the use of local anesthetics may be related to the total dose of local anesthetic administered and are also dependent upon the particular drug used, the route of administration, and the physical status of the patient. Many of these effects may be related to local anesthetic techniques, with or without a contribution from the drug. Neurologic effects following infiltration of soft tissue may include persistent anesthesia, paresthesia, weakness, and paralysis, all of which may have slow, incomplete, or no recovery.
Central nervous system reactions are characterized by excitation and/or depression. Restlessness, anxiety, dizziness, tinnitus, blurred vision, or tremors may occur, possibly proceeding to convulsions. However, excitement may be transient or absent, with depression being the first manifestation of an adverse reaction. This may quickly be followed by drowsiness merging into unconsciousness and respiratory arrest. Other central nervous system effects may be nausea, vomiting, chills, and constriction of the pupils. The incidence of convulsions associated with the use of local anesthetics varies with the procedure used and the total dose administered.
Cardiovascular System Reactions
Toxic blood concentrations depress cardiac conductivity and excitability, which may lead to atrioventricular block, ventricular arrhythmias, and cardiac arrest, sometimes resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure [See Overdosage (10)].
Allergic Reactions
Allergic-type reactions are rare and may occur as a result of hypersensitivity to the local anesthetic or to other formulation ingredients. These reactions are characterized by signs such as urticaria, pruritus, erythema, angioneurotic edema (including laryngeal edema), tachycardia, sneezing, nausea, vomiting, dizziness, syncope, excessive sweating, elevated temperature, and possibly anaphylactoid-like symptoms (including severe hypotension). Cross-sensitivity among members of the amide-type local anesthetic group has been reported. The usefulness of screening for sensitivity has not been definitively established.
Chondrolysis
Intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures is an unapproved use, and there have been postmarketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric patients and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are not associated with these findings. The time of onset of symptoms, such as joint pain, stiffness, and loss of motion can be variable, but may begin as early as the second month after surgery. Currently, there is no effective treatment for chondrolysis; patients who have experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue EXPAREL and any oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
5.2 Warnings and Precautions Specific for EXPAREL
As there is a potential risk of severe life-threatening adverse reactions associated with the administration of bupivacaine, EXPAREL should be administered in a setting where trained personnel and equipment are available to promptly treat patients who show evidence of neurological or cardiac toxicity [See Overdosage (10)].
Caution should be taken to avoid accidental intravascular injection of EXPAREL. Convulsions and cardiac arrest have occurred following accidental intravascular injection of bupivacaine and other amide-containing products.
Avoid additional use of local anesthetics within 96 hours following administration of EXPAREL [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
EXPAREL has not been evaluated for the following uses and, therefore, is not recommended for these routes of administration or types of analgesia:
- epidural
- intrathecal
- intravascular or intra-articular use
- regional nerve blocks other than interscalene brachial plexus nerve block, sciatic nerve block in the popliteal fossa, and adductor canal block
EXPAREL has not been evaluated for use in the following patient populations and, therefore, is not recommended for administration to these groups.
- patients younger than 6 years old for infiltration
- patients younger than 18 years old for interscalene brachial plexus nerve block, sciatic nerve block in the popliteal fossa, and adductor canal block
- pregnant patients
The potential sensory and/or motor loss with EXPAREL is temporary and varies in degree and duration depending on the site of injection and dose administered and may last for up to 5 days as seen in clinical trials.
-
6 ADVERSE REACTIONS
The following serious adverse reactions have been associated with bupivacaine hydrochloride in clinical trials and are described in greater detail in other sections of the labeling:
- Central Nervous System Reactions [see Warnings and Precautions (5.1)]
- Cardiovascular System Reactions [see Warnings and Precautions (5.1)]
- Allergic Reactions [see Warnings and Precautions (5.1)]
- Chondrolysis [see Warnings and Precautions (5.1)]
- Methemoglobinemia [see Warnings and Precautions (5.1)]
- Accidental intravascular injection [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adverse Reactions Reported in All Local Infiltration Clinical Studies in Adults
The safety of EXPAREL (local administration into the surgical site) was evaluated in 10 randomized, double-blind, clinical studies (including Studies 1 and 2 [see Clinical Studies (14.2)]) that included 823 adult patients who had various surgical procedures. Patients were administered an EXPAREL dose ranging from 66 to 532 mg (two times the maximum recommended dose of 266 mg). In these studies, following EXPAREL administration, the:
- Most common adverse reactions (incidence greater than or equal to 10%) were nausea, constipation, and vomiting.
- Common adverse reactions (incidence greater than or equal to 2% to less than 10%) were pyrexia, dizziness, peripheral edema, anemia, hypotension, pruritus, tachycardia, headache, insomnia, postoperative anemia, muscle spasms, hemorrhagic anemia, back pain, somnolence, and procedural pain.
- Less common adverse reactions (incidence less than 2%) were chills, erythema, bradycardia, anxiety, urinary retention, pain, edema, tremor, postural dizziness, paresthesia, syncope, incision site edema, procedural hypertension, procedural hypotension, procedural nausea, muscular weakness, neck pain, generalized pruritus, pruritic rash, hyperhidrosis, cold sweat, urticaria, palpitations, sinus bradycardia, supraventricular extrasystoles, ventricular extrasystoles, ventricular tachycardia, hypertension, pallor, anxiety, confusional state, depression, agitation, restlessness, hypoxia, laryngospasm, apnea, respiratory depression, respiratory failure, increased body temperature, increased blood pressure (BP), decreased BP, decreased oxygen saturation, urinary incontinence, blurred vision, tinnitus, drug hypersensitivity, and hypersensitivity.
Neurological and Cardiac Adverse Reactions
In the EXPAREL surgical site infiltration studies, following EXPAREL administration adverse reactions with an incidence greater than or equal to 1% in the:
- Nervous System Disorders system organ class were dizziness (6.2%), headache (3.8%), somnolence (2.1%), hypoesthesia (1.5%), and lethargy (1.3%).
- Cardiac Disorders system organ class were tachycardia (3.9%) and bradycardia (1.6%).
Adverse Reactions Reported in All Local Infiltration Placebo-Controlled Trials in Adults
Adverse reactions with an incidence greater than or equal to 2% reported by adult patients in clinical studies who underwent a bunionectomy (Study 1) or hemorrhoidectomy (Study 2) [see Clinical Studies (14.2)] that compared 106 mg of EXPAREL (8 mL) to placebo and 266 mg of EXPAREL (20 mL) to placebo are shown in Table 1.
Table 1: Treatment-Emergent Adverse Reactions with an Incidence Greater than or Equal to 2%: Local Infiltration Placebo-Controlled Studies in Adults (Studies 1 and 2) System Organ Class
Preferred TermStudy 1* Study 2† EXPAREL Placebo EXPAREL Placebo 106 mg
(N=97)
n (%)(N=96)
n (%)266 mg
(N=95)
n (%)(N=94)
n (%)TEAE = treatment-emergent adverse event.
At each level of summation (overall, system organ class, preferred term), patients are only counted once. Preferred terms are included where at least 2% of patients reported the event in any treatment group.Any TEAE 53 (54.6) 59 (61.5) 10 (10.5) 17 (18.1) Gastrointestinal Disorders 41 (42.3) 38 (39.6) 7 (7.4) 13 (13.8) Nausea 39 (40.2) 36 (37.5) 2 (2.1) 1 (1.1) Vomiting 27 (27.8) 17 (17.7) 2 (2.1) 4 (4.3) Constipation 2 (2.1) 1 (1.0) 2 (2.1) 2 (2.1) Anal Hemorrhage 0 (0.0) 0 (0.0) 3 (3.2) 4 (4.3) Painful Defecation 0 (0.0) 0 (0.0) 2 (2.1) 5 (5.3) Rectal Discharge 0 (0.0) 0 (0.0) 1 (1.1) 3 (3.2) Nervous System Disorders 20 (20.6) 30 (31.3) 0 (0.0) 0 (0.0) Dizziness 11 (11.3) 25 (26.0) 0 (0.0) 0 (0.0) Headache 5 (5.2) 8 (8.3) 0 (0.0) 0 (0.0) Somnolence 5 (5.2) 1 (1.0) 0 (0.0) 0 (0.0) Syncope 2 (2.1) 0 (0.0) 0 (0.0) 0 (0.0) Skin And Subcutaneous Tissue Disorders 8 (8.2) 7 (7.3) 0 (0.0) 0 (0.0) Generalized Pruritus 5 (5.2) 6 (6.3) 0 (0.0) 0 (0.0) Pruritus 3 (3.1) 1 (1.0) 0 (0.0) 0 (0.0) Investigations 5 (5.2) 3 (3.1) 4 (4.2) 3 (3.2) Increased Alanine Aminotransferase 3 (3.1) 3 (3.1) 1 (1.1) 0 (0.0) Increased Aspartate Aminotransferase 3 (3.1) 2 (2.1) 0 (0.0) 0 (0.0) Increased Blood Creatinine 2 (2.1) 0 (0.0) 0 (0.0) 0 (0.0) Increased Body Temperature 0 (0.0) 0 (0.0) 3 (3.2) 3 (3.2) General Disorders And Administration Site Conditions 4 (4.1) 0 (0.0) 1 (1.1) 1 (1.1) Feeling Hot 2 (2.1) 0 (0.0) 0 (0.0) 0 (0.0) Pyrexia 2 (2.1) 0 (0.0) 1 (1.1) 1 (1.1) Infections And Infestations 2 (2.1) 1 (1.0) 0 (0.0) 0 (0.0) Fungal Infection 2 (2.1) 1 (1.0) 0 (0.0) 0 (0.0) Injury, Poisoning And Procedural Complications 2 (2.1) 0 (0.0) 0 (0.0) 0 (0.0) Post Procedural Swelling 2 (2.1) 0 (0.0) 0 (0.0) 0 (0.0) Metabolism And Nutrition Disorders 2 (2.1) 2 (2.1) 0 (0.0) 0 (0.0) Decreased Appetite 2 (2.1) 2 (2.1) 0 (0.0) 0 (0.0) Adverse Reactions Reported in All Local Infiltration Clinical Studies in Pediatric Patients Aged 6 to Less Than 17 Years
The safety of EXPAREL in 110 pediatric patients between the age of 6 and 17 years old who had spine or cardiac surgical procedures was evaluated in one randomized, open-label, clinical study in which EXPAREL was administered by infiltration into the surgical site (Study Peds-1) and one single-arm, open-label study in which EXPAREL was administered by infiltration into the surgical site (Study Peds-2) [see Use in Specific Populations (8.4)]. Patients were administered a weight-based dose of EXPAREL at 4 mg/kg (maximum dose of 266 mg) or bupivacaine HCl 2 mg/kg (maximum dose of 175 mg).
In these studies, following EXPAREL administration the:
- Most common adverse reactions (incidence greater than or equal to 10%) were nausea, vomiting, constipation, hypotension, anemia, muscle twitching, blurred vision, pruritus, and tachycardia.
- Common adverse reactions (incidence greater than or equal to 2% to less than 10%) were bradycardia, muscle spasms, tachypnea, oral hypoesthesia, postoperative anemia, dizziness, pyrexia, diarrhea, hypoacusis, hypoesthesia, back pain, hematuria, incontinence, muscular weakness, and visual impairment.
- Less common adverse reactions (incidence less than 2%) were flatulence, abdominal pain, dyspepsia, lip swelling, pain in extremity, musculoskeletal pain, flank pain, musculoskeletal chest pain, hypertension, sinus tachycardia, ventricular extrasystoles, dysgeusia, paresthesia, burning sensation, syncope, diplopia, eye swelling, dyspnea, atelectasis, hypopnea, hypoxia, chest pain, face edema, gait disturbance, generalized pruritus, rash, delayed recovery from anesthesia, fall, incision site hemorrhage, joint dislocation, seroma, hypomagnesemia, acidosis, hyperglycemia, metabolic acidosis, ear discomfort, decreased urine output, increased heart rate (HR), anxiety, panic attack, ear infection, and fungal wound infection.
Neurological and Cardiac Adverse Reactions in Pediatric Patients Aged 6 to Less than 17 Years Old
In the EXPAREL infiltration studies in pediatric patients aged 6 to less than 17 years old (Studies Peds-1 and Peds-2), following EXPAREL administration adverse reactions with an incidence greater than or equal to 1% in the:
- Nervous System Disorders system organ class were dizziness (6.3%, n=5), and dysgeusia (1.3%, n=1).
- Cardiac Disorders system organ class were tachycardia (11.3%, n=9), bradycardia (8.8%, n=7), sinus tachycardia (1.3%, n=1), and ventricular extrasystoles (1.3%, n=1).
Adverse Reactions Reported in All Local Infiltration Trials in Pediatric Patients Aged 6 to Less than 17 Years Old
Adverse reactions with an incidence greater than or equal to 2% reported by patients in clinical studies (Studies Peds-1 and Peds-2) studying 4 mg/kg EXPAREL are shown in Table 2.
Table 2: Treatment-Emergent Adverse Reactions (TEAE) with an Incidence Greater than or Equal to 2%: Local Infiltration Studies in Pediatric Patients Aged 6 to Less than 17 Years Old (Study Peds-1 and Peds-2) System Organ Class
Preferred TermStudy Peds-1* Study Peds-2† Spine Surgery
EXPAREL
4 mg/kg‡
(N=36)
n (%)Cardiac Surgery
EXPAREL
4 mg/kg‡
(N=29)
n (%)Spine Surgery
EXPAREL
4 mg/kg‡
(N=15)
n (%)At each level of summation (overall, system organ class, preferred term), patients are only counted once. Preferred terms are included where at least 2% of patients reported the event in any treatment group.
TEAE = treatment-emergent adverse event.Patients with at least one TEAE 24 (66.7) 9 (31.0) 15 (100.0) Blood and lymphatic system disorders 0 0 15 (100) Anemia 0 0 15 (100) Cardiac disorders 3 (8.3) 1 (3.4) 12 (80.0) Bradycardia 2 (5.6) 0 5 (33.3) Sinus tachycardia 0 1 (3.4) 0 Tachycardia 1 (2.8) 0 8 (53.3) Ventricular extrasystoles 0 0 1 (6.7) Ear and labyrinth disorders 2 (5.6) 0 2 (13.3) Ear discomfort 0 0 1 (6.7) Hypoacusis 2 (5.6) 0 1 (6.7) Eye disorders 10 (27.8) 1 (3.4) 4 (26.7) Diplopia 1 (2.8) 0 0 Eye swelling 0 0 1 (6.7) Increased Lacrimation 0 0 0 Blurred Vision 7 (19.4) 1 (3.4) 3 (20.0) Visual impairment 2 (5.6) 0 0 Gastrointestinal disorders 18 (50.0) 7 (24.1) 14 (93.3) Abdominal Pain 0 0 1 (6.7) Constipation 9 (25.0) 4 (13.8) 7 (46.7) Nausea 11 (30.6) 2 (6.9) 9 (60.0) Diarrhea 3 (8.3) 0 0 Dyspepsia 1 (2.8) 0 0 Flatulence 0 0 1 (6.7) Oral Hypoesthesia 4 (11.1) 0 2 (13.3) Lip Swelling 0 0 1 (6.7) Vomiting 10 (27.8) 4 (13.8) 8 (53.3) General disorders and administration site conditions 0 1 (3.4) 3 (20.0) Chest pain 1 (2.8) 0 0 Face edema 0 1 (3.4) 0 Gait disturbance 0 0 1 (6.7) Generalized edema 0 0 0 Pyrexia 0 0 3 (20.0) Infections and infestations 1 (2.8) 1 (3.4) 0 Ear infection 1 (2.8) 0 0 Fungal wound infection 0 1 (3.4) 0 Injury, poisoning and procedural complications 8 (22.2) 0 1 (6.7) Postoperative Anemia 5 (13.9) 0 0 Delayed recovery from anesthesia 1 (2.8) 0 0 Fall 0 0 1 (6.7) Incision site hemorrhage 1 (2.8) 0 0 Joint dislocation 1 (2.8) 0 0 Procedural hemorrhage 0 0 0 Seroma 1 (2.8) 0 0 Metabolism and nutrition disorders 0 3 (10.3) 0 Acidosis 0 1 (3.4) 0 Hyperglycemia 0 1 (3.4) 0 Hypomagnesaemia 0 1 (3.4) 0 Metabolic acidosis 0 1 (3.4) 0 Musculoskeletal and connective tissue disorders 8 (22.2) 1 (3.4) 12 (80.0) Back pain 0 0 2 (13.3) Flank pain 0 0 1 (6.7) Muscle twitching 3 (8.3) 1 (3.4) 9 (60.0) Muscle spasms 4 (11.1) 0 3 (20.0) Muscular weakness 0 0 2 (13.3) Musculoskeletal pain 1 (2.8) 0 0 Musculoskeletal chest pain 0 0 1 (6.7) Pain in extremity 0 0 1 (6.7) Nervous system disorders 3 (8.3) 0 7 (46.7) Burning sensation 0 0 1 (6.7) Dizziness 2 (5.6) 0 3 (20.0) Dysgeusia 1 (2.8) 0 0 Headache 0 0 0 Hypoesthesia 0 0 3 (20.0) Paresthesia 0 0 1 (6.7) Syncope 1 (2.8) 0 0 Psychiatric disorders 0 2 (13.3) Anxiety 0 0 1 (6.7) Panic attack 0 0 1 (6.7) Renal and urinary disorders 0 0 2 (13.3) Hematuria 0 0 2 (13.3) Respiratory, thoracic and mediastinal disorders 3 (8.3) 1 (3.4) 7 (46.7) Atelectasis 0 0 1 (6.7) Bradypnea 0 0 0 Dyspnea 0 1 (3.4) 0 Hypopnea 1 (2.8) 0 0 Hypoxia 1 (2.8) 0 0 Pleural effusion 0 0 0 Tachypnea 1 (2.8) 0 6 (40.0) Skin and subcutaneous tissue disorders 4 (11.1) 0 6 (40.0) Pruritus 3 (8.3) 0 6 (40.0) Generalized Pruritus 1 (2.8) 0 0 Rash 0 0 1 (6.7) Vascular disorders 4 (11.1) 1 (3.4) 14 (93.3) Hot flush 0 0 0 Hypotension 4 (11.1) 0 14 (93.3) Hypertension 0 1 (3.4) 0 Systolic hypertension 0 0 0 Adverse Reactions Reported in Placebo-Controlled Nerve Block Clinical Studies in Adults
The safety of EXPAREL was evaluated in four randomized, double-blind, placebo-controlled nerve block clinical studies (Studies 3, 6, 7, 8) [see Clinical Studies (14.3, 14.4)] involving 469 EXPAREL-treated adult patients and 357 placebo-treated patients who had various surgical procedures. Patients were administered placebo or an EXPAREL dose of either 133 or 266 mg (two times the maximum recommended dose for these nerve blocks). In these studies, following EXPAREL administration via nerve block (perineural use) the:
- Most common adverse reactions (incidence greater than or equal to 10%) were nausea, pyrexia, and constipation.
- Common adverse reactions (incidence greater than or equal to 2% to less than 10%) were muscle twitching, dysgeusia, urinary retention, fatigue, headache, confusional state, hypotension, hypertension, oral hypoesthesia, generalized pruritus, hyperhidrosis, tachycardia, sinus tachycardia, anxiety, fall, increased body temperature, peripheral edema, sensory loss, increased hepatic enzyme, hiccups, hypoxia, and post-procedural hematoma.
- Less common adverse reactions (incidence less than 2%) were arrhythmia, atrial fibrillation, first degree atrioventricular block, bradycardia, left bundle branch block, right bundle branch block, cardiac arrest, impaired hearing, blurred vision, visual impairment, asthenia, chills, hyperthermia, cellulitis, lung infection, pneumonia, procedural nausea, wound dehiscence, wound secretion, electrocardiogram QT prolonged, white blood cell count increased, arthralgia, back pain, joint swelling, decreased mobility, muscle spasms, muscular weakness, musculoskeletal pain, paraesthesia, presyncope, sedation, somnolence, syncope, delirium, dysuria, urinary incontinence, atelectasis, cough, dyspnea, lung infiltration, blister, drug eruption, erythema, rash, urticaria, deep vein thrombosis, hematoma, and orthostatic hypotension.
The most common and common adverse reactions for the four randomized, double-blind, placebo-controlled nerve block clinical studies (Studies 3, 6, 7, 8) are shown in Table 3.
Neurological and Cardiac Adverse Reactions
In the EXPAREL nerve block placebo-controlled studies, following EXPAREL administration adverse reactions with an incidence greater than or equal to 1% in the:
- Nervous System Disorders system organ class were motor dysfunction (14.9%), dysgeusia (7.2%), headache (5.1%), hypoesthesia (2.3%), and sensory loss (2.3%).
- Cardiac Disorders system organ class were tachycardia (3%), sinus tachycardia (2.3%), and bradycardia (1.3%).
Table 3: Treatment-Emergent Adverse Reactions with an Incidence Greater than or Equal to 2%: Nerve Block Placebo-Controlled Studies (Studies 3, 6, 7, and 8) SYSTEM ORGAN CLASS
Preferred TermEXPAREL
133 mg
(N=168)
n (%)EXPAREL
266 mg
(N=301)
n (%)Placebo
(N=357)
n (%)At each level of summation (overall, system organ class, preferred term), patients are only counted once.
Preferred terms are included where at least 2% of patients reported the event in any treatment group.
TEAE = treatment-emergent adverse event.Number of Patients with at Least One TEAE 152 (90.5) 260 (86.4) 299 (83.8) Blood and Lymphatic System Disorders 2 (1.2) 22 (7.3) 15 (4.2) Anemia 2 (1.2) 18 (6.0) 13 (3.6) Cardiac Disorders 13 (7.7) 34 (11.3) 38 (10.6) Atrial Fibrillation 1 (0.6) 4 (1.3) 8 (2.2) Sinus Tachycardia 3 (1.8) 8 (2.7) 4 (1.1) Tachycardia 3 (1.8) 11 (3.7) 10 (2.8) Gastrointestinal Disorders 84 (50.0) 154 (51.2) 184 (51.5) Constipation 29 (17.3) 66 (21.9) 68 (19.0) Dyspepsia 3 (1.8) 7 (2.3) 7 (2.0) Oral Hypoesthesia 6 (3.6) 8 (2.7) 7 (2.0) Nausea 62 (36.9) 111 (36.9) 133 (37.3) Vomiting 17 (10.1) 55 (18.3) 73 (20.4) General Disorders And Administration Site Conditions 52 (31.0) 102 (33.9) 91 (25.5) Fatigue 7 (4.2) 15 (5.0) 15 (4.2) Feeling Cold 0 10 (3.3) 8 (2.2) Peripheral Edema 4 (2.4) 6 (2.0) 8 (2.2) Peripheral Swelling 3 (1.8) 8 (2.7) 4 (1.1) Pyrexia 36 (21.4) 70 (23.3) 64 (17.9) Injury, Poisoning And Procedural Complications 18 (10.7) 44 (14.6) 32 (9.0) Postoperative Anemia 0 8 (2.7) 10 (2.8) Contusion 4 (2.4) 1 (0.3) 0 Fall 4 (2.4) 8 (2.7) 1 (0.3) Post Procedural Hematoma 4 (2.4) 1 (0.3) 0 Procedural Hypotension 2 (1.2) 13 (4.3) 7 (2.0) Investigations 18 (10.7) 31 (10.3) 31 (8.7) Increased Body Temperature 1 (0.6) 10 (3.3) 4 (1.1) Increased Hepatic Enzyme 7 (4.2) 1 (0.3) 3 (0.8) Metabolism and Nutrition Disorders 13 (7.7) 18 (6.0) 25 (7.0) Hypokalemia 7 (4.2) 9 (3.0) 14 (3.9) Musculoskeletal And Connective Tissue Disorders 22 (13.1) 47 (15.6) 41 (11.5) Decreased Mobility 0 6 (2.0) 5 (1.4) Muscle Twitching 14 (8.3) 21 (7.0) 25 (7.0) Nervous System Disorders 72 (42.9) 101 (33.6) 112 (31.4) Dizziness 8 (4.8) 28 (9.3) 40 (11.2) Dysgeusia 12 (7.1) 22 (7.3) 21 (5.9) Headache 14 ( 8.3) 10 (3.3) 10 (2.8) Hypoesthesia 6 (3.6) 5 (1.7) 2 (0.6) Motor Dysfunction 35 (20.8) 35 (11.6) 37 (10.4) Sensory Loss 4 (2.4) 7 (2.3) 1 (0.3) Psychiatric Disorders 10 (6.0) 33 (11.0) 44 (12.3) Anxiety 3 (1.8) 9 (3.0) 6 (1.7) Confusional State 3 (1.8) 15 (5.0) 14 (3.9) Insomnia 5 (3.0) 10 (3.3) 19 (5.3) Renal And Urinary Disorders 9 (5.4) 31 (10.3) 31 (8.7) Urinary Retention 5 (3.0) 23 (7.6) 22 (6.2) Respiratory, Thoracic And Mediastinal Disorders 18 (10.7) 30 (10.0) 31 (8.7) Dyspnea 2 (1.2) 4 (1.3) 8 (2.2) Hiccups 4 (2.4) 4 (1.3) 1 (0.3) Hypoxia 4 (2.4) 3 (1.0) 3 (0.8) Skin And Subcutaneous Tissue Disorders 24 (14.3) 63 (20.9) 84 (23.5) Hyperhidrosis 1 (0.6) 14 (4.7) 15 (4.2) Pruritus 10 (6.0) 45 (15.0) 55 (15.4) Generalized Pruritus 6 (3.6) 7 (2.3) 14 (3.9) Vascular Disorders 16 (9.5) 30 (10.0) 44 (12.3) Hypertension 3 (1.8) 15 (5.0) 21 (5.9) Hypotension 11 (6.5) 8 (2.7) 19 (5.3) Adverse Reactions Reported in Active-Controlled Nerve Block Clinical Studies in Approved Populations
The safety of EXPAREL was evaluated in two randomized, double-blind, active-controlled nerve block clinical studies in 189 adult patients who had a bunionectomy or a total knee arthroplasty (Studies 4 and 5) [see Clinical Studies (14.3)]. Via nerve block, patients received 133 mg of EXPAREL, 266 mg of EXPAREL (two times the maximum recommended EXPAREL dose) [see Dosage and Administration (2.3)] or 133 mg of EXPAREL admixed with 50 mg of bupivacaine HCl. In both of these studies the active comparator was 50 mg of bupivacaine HCl.
The most common adverse reactions (incidence greater than or equal to 10%) in Studies 4 and 5 following:
- EXPAREL administration as a nerve block were nausea and constipation.
- Administration of EXPAREL admixed with bupivacaine as a nerve block were nausea, constipation, muscle spasms, and headache.
The common adverse reactions (incidence greater than or equal to 2% to less than 10%) in Studies 4 and 5 following:
- EXPAREL administration as a nerve block were pruritus, vomiting, dyspepsia, headache, peroneal nerve palsy, rash and hypertension.
- Administration of EXPAREL admixed with bupivacaine as a nerve block were vomiting, dyspepsia, heart rate increased, hypokalaemia, hyponatraemia, back pain, disorientation, oropharyngeal pain, hypoaesthesia, pruritus, dizziness, insomnia, hypertension, hypoxia, hypotension, pyrexia, and tachycardia.
The less common adverse reactions (incidence less than 2%) in Studies 4 and 5 following:
- EXPAREL administration as a nerve block were increased BP, pyrexia, arthralgia, insomnia, muscle spasms, asthenia, increased systolic BP, diarrhea, facial pain, migraine, muscle twitching, throat irritation, post procedural erythema, post procedural edema, dizziness, hypoesthesia, rhinorrhea, and paresthesia.
- Administration of EXPAREL admixed with bupivacaine as a nerve block were increased BP, procedural pain, rash, pruritic rash, anemia, anxiety, arthralgia, atrial fibrillation, decreased blood potassium, decreased BP, increased systolic BP, increased body temperature, bradycardia, confusional state, decreased appetite, diarrhea, dysarthria, fall, feeling cold, decreased HR, hot flush, joint swelling, leukocytosis, mental status changes, neuralgia, orthostatic hypotension, decreased oxygen saturation, pneumonia, post procedural hematoma, post procedural inflammation, post procedural swelling, pruritic rash, positive staphylococcus test, syncope, and urinary tract infection .
The most common and common adverse reactions in adult patients in the active-controlled clinical studies are shown in Table 4.
Table 4: Treatment-Emergent Adverse Reactions with an Incidence Greater than or Equal to 2%: Nerve Block Active-Controlled Studies (Studies 4 and 5) SYSTEM ORGAN CLASS
Preferred TermEXPAREL
133 mg
(N=81)
n (%)EXPAREL
266 mg
(N=22)
n (%)EXPAREL
133 mg + Bupi
(N = 86)
n (%)Bupi
(N=162)
n (%)At each level of summation (overall, system organ class, preferred term), patients were only counted once.
Preferred terms are included where at least 2% of patients reported the event in any treatment group.
TEAE = treatment-emergent adverse event.Number of Patients with at Least One TEAE 42 (51.9) 13 (59.1) 77 (89.5) 116 (71.6) Cardiac Disorders Tachycardia 0 0 4 (4.7) 5 (3.1) Gastrointestinal Disorders Constipation 10 (12.3) 3 (13.6) 30 (34.9) 47 (29.0) Dyspepsia 2 (2.5) 1 (4.5) 2 (2.3) 2 (1.2) Nausea 13 (16) 9 (40.9) 34 (39.5) 49 (30.2) Vomiting 4 (4.9) 5 (22.7) 5 (5.8) 13 (8.0) General Disorders And Administration Site Conditions Pyrexia 0 1 (4.5) 3 (3.5) 3 (1.9) Investigations Increased Heart rate 0 0 3 (3.5) 3 (1.9) Metabolism and Nutrition Disorders Hypokalemia 0 0 2 (2.3) 2 (1.2) Hyponatremia 0 0 2 (2.3) 2 (1.2) Musculoskeletal And Connective Tissue Disorders Back pain 0 0 2 (2.3) 3 (1.9) Muscle Spasms 2 (2.5) 0 11 (12.8) 10 (6.2) Nervous System Disorders Dizziness 2 (2.5) 0 4 (4.7) 4 (2.5) Headache 8 (9.9) 1 (4.5) 13 (15.1) 6 (3.7) Hypoaesthesia 0 9 (12.7) 2 (2.1) 0 Peroneal Nerve Palsy 3 (3.7) 0 0 0 Psychiatric Disorders Disorientation 0 0 2 (2.3) 0 Insomnia 2 (2.5) 0 5 (5.8) 14 (8.6) Respiratory, Thoracic And Mediastinal Disorders Hypoxia 0 0 4 (4.7) 4 (2.5) Oropharyngeal Pain 0 0 2 (2.3) 0 Skin And Subcutaneous Tissue Disorders Pruritus 6 (7.4) 1 (4.5) 6 (7.0) 9 (5.6) Rash 4 (4.9) 1 (4.5) 1 (1.2) 1 (0.6) Vascular Disorders Hypertension 3 (3.7) 1 (4.5) 5 (5.8) 7 (4.3) Hypotension 0 0 3 (3.5) 9 (5.6) Notable Adverse Reactions from Active-Controlled Studies in Unapproved Populations
The safety of EXPAREL was evaluated in a multicenter, randomized, double-blind, active-controlled trial in 119 patients undergoing foot and ankle procedures (Study 9) [see Clinical Studies (14.3)]. Patients were administered a dose of either 266 mg EXPAREL, 266 mg EXPAREL admixed with 50 mg bupivacaine HCl, or 100 mg bupivacaine HCl. The following adverse reactions were observed following administration of EXPAREL or EXPAREL admixed with bupivacaine in Study 9 that either were not observed in Studies 4 and 5, or were observed at a higher frequency than was observed in Studies 4 and 5. These include:
- The most common adverse reactions (incidence greater than 10%) observed in Study 9 at higher frequencies than Studies 4 and 5: hypoesthesia (21% vs. 1% and 0%, respectively), paresthesia (10% vs. 1% and 0%, respectively).
- The common adverse reactions (incidence greater than or equal to 2% to less than 10%) observed in Study 9 but not in Studies 4 and 5: epistaxis, motor dysfunction, pain in extremity, skin abrasion, infusion site pain, muscular weakness.
6.2 Postmarketing Experience
Because adverse reactions reported during postmarketing are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These adverse reactions are consistent with those observed in clinical studies and most commonly involve the following system organ classes: Injury, Poisoning, and Procedural Complications (e.g., drug-drug interaction, procedural pain), Nervous System Disorders (e.g., palsy, seizure), General Disorders And Administration Site Conditions (e.g., lack of efficacy, pain), Skin And Subcutaneous Tissue Disorders (e.g., erythema, rash), and Cardiac Disorders (e.g., bradycardia, cardiac arrest).
-
7 DRUG INTERACTIONS
The toxic effects of local anesthetics are additive and concomitant use should be used with caution including monitoring for neurologic and cardiovascular effects related to local anesthetic systemic toxicity [see Dosage and Administration (2.2), Warnings and Precautions (5.1), and Overdosage (10)]. Avoid additional use of local anesthetics within 96 hours following administration of EXPAREL.
Patients who are administered local anesthetics, including EXPAREL, may be at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Class Examples Nitrates/Nitrites nitric oxide, nitroglycerin, nitroprusside, nitrous oxide Local anesthetics articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine Antineoplastic agents cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase Antibiotics dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides Antimalarials chloroquine, primaquine Anticonvulsants Phenobarbital, phenytoin, sodium valproate Other drugs acetaminophen, metoclopramide, quinine, sulfasalazine Bupivacaine
Bupivacaine HCl administered together with EXPAREL may impact the pharmacokinetic and/or physicochemical properties of EXPAREL, and this effect is concentration dependent. Therefore, bupivacaine HCl and EXPAREL may be administered simultaneously in the same syringe, and bupivacaine HCl may be injected immediately before EXPAREL as long as the ratio of the milligram dose of bupivacaine HCl solution to EXPAREL does not exceed 1:2.
Non-Bupivacaine Local Anesthetics
EXPAREL should not be admixed with local anesthetics other than bupivacaine. Non-bupivacaine based local anesthetics, including lidocaine, may cause an immediate release of bupivacaine from EXPAREL if administered together locally. The administration of EXPAREL may follow the administration of lidocaine after a delay of 20 minutes or more. There are no data to support administration of other local anesthetics prior to administration of EXPAREL.
Other than bupivacaine as noted above, EXPAREL should not be admixed with other drugs prior to administration.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no studies conducted with EXPAREL in pregnant women. In animal reproduction studies, embryo-fetal deaths were observed with subcutaneous administration of bupivacaine to rabbits during organogenesis at a dose equivalent to 1.6 times the maximum recommended human dose (MRHD) of 266 mg. Subcutaneous administration of bupivacaine to rats from implantation through weaning produced decreased pup survival at a dose equivalent to 1.5 times the MRHD [see Data]. Based on animal data, advise pregnant women of the potential risks to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
Clinical Considerations
Labor or Delivery
Bupivacaine is contraindicated for obstetrical paracervical block anesthesia. While EXPAREL has not been studied with this technique, the use of bupivacaine for obstetrical paracervical block anesthesia has resulted in fetal bradycardia and death.
Bupivacaine can rapidly cross the placenta, and when used for epidural, caudal, or pudendal block anesthesia, can cause varying degrees of maternal, fetal, and neonatal toxicity [See Clinical Pharmacology (12.3)]. The incidence and degree of toxicity depend upon the procedure performed, the type, and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus, and neonate involve alterations of the central nervous system, peripheral vascular tone, and cardiac function.
Data
Animal Data
Bupivacaine hydrochloride was administered subcutaneously to rats and rabbits during the period of organogenesis (implantation to closure of the hard plate). Rat doses were 4.4, 13.3, and 40 mg/kg/day (equivalent to 0.2, 0.5 and 1.5 times the MRHD, respectively, based on the BSA comparisons and a 60 kg human weight) and rabbit doses were 1.3, 5.8, and 22.2 mg/kg/day (equivalent to 0.1, 0.4 and 1.6 times the MRHD, respectively, based on the BSA comparisons and a 60 kg human weight). No embryo-fetal effects were observed in rats at the doses tested with the high dose causing increased maternal lethality. An increase in embryo-fetal deaths was observed in rabbits at the high dose in the absence of maternal toxicity.
Decreased pup survival was noted at 1.5 times the MRHD in a rat pre- and post-natal development study when pregnant animals were administered subcutaneous doses of 4.4, 13.3, and 40 mg/kg/day bupivacaine hydrochloride (equivalent to 0.2, 0.5 and 1.5 times the MRHD, respectively, based on the BSA comparisons and a 60 kg human weight) from implantation through weaning (during pregnancy and lactation).
8.2 Lactation
Risk Summary
Limited published literature reports that bupivacaine and its metabolite, pipecoloxylidide, are present in human milk at low levels. There is no available information on effects of the drug in the breastfed infant or effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EXPAREL and any potential adverse effects on the breastfed infant from EXPAREL or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of EXPAREL to produce postsurgical local analgesia via infiltration have been established in pediatric patients aged 6 years and older. Use of EXPAREL for this indication is supported by evidence from adequate and well-controlled studies in adults, and pharmacokinetic (PK) and safety data in pediatric patients aged 6 years and older from Studies Peds-1 and Peds-2 [see Adverse Reactions (6.1), Clinical Pharmacology (12.3)].
- Study Peds-1 was a multicenter, randomized, open-label, two-part study (NCT03682302) to evaluate the PK and safety of EXPAREL for local infiltration in pediatric patients aged 6 to less than 17 years who were undergoing spine or cardiac surgery (postsurgically, patients were administered opioid rescue medication according to the study site's standard of care).
- Group 1: 61 patients aged 12 to less than 17 years, undergoing spine surgeries, were randomized 1:1 to receive either EXPAREL 4 mg/kg (maximum 266 mg) or bupivacaine HCl 2 mg/kg (maximum 175 mg).
- Group 2: 34 patients aged 6 to less than 12 years, undergoing either spine or cardiac surgeries, received open-label EXPAREL 4 mg/kg (maximum up to 266 mg).
- Study Peds-2 was a phase 1, open-label study that evaluated the PK and safety of 4 mg/kg (maximum 266 mg) of EXPAREL (administered intraoperatively prior to wound closure) in 15 pediatric patients aged 12 to less than 17 who were undergoing spinal surgery.
The safety and effectiveness of EXPAREL have not been established to produce postsurgical:
- Local analgesia via infiltration in pediatric patients aged less than 6 years old.
- Regional analgesia via an interscalene brachial plexus nerve block, sciatic nerve block in the popliteal fossa, or adductor canal block in pediatric patients.
8.5 Geriatric Use
Of the total number of patients in the EXPAREL local infiltration clinical studies (N=823), 171 patients were greater than or equal to 65 years of age and 47 patients were greater than or equal to 75 years of age. Of the total number of patients in the EXPAREL nerve block clinical studies (N= 1046), 312 patients were greater than or equal to 65 years of age and 70 patients were greater than or equal to 75 years of age. No overall differences in safety or effectiveness of EXPAREL have been observed between patients 65 years of age and older and younger adult patients.
In clinical studies, differences in various pharmacokinetic parameters have been observed between patients 65 years of age and older and younger adult patients. Bupivacaine is known to be substantially excreted by the kidney, and the risk of adverse reactions to bupivacaine may be greater in patients with renal impairment than in patients with normal renal function. Because patients 65 years of age and older are more likely to have renal impairment, increase monitoring for EXPAREL-associated adverse reactions [see Adverse Reactions (6)].
8.6 Hepatic Impairment
Amide-type local anesthetics, such as bupivacaine, are metabolized by the liver. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations, and potentially local anesthetic systemic toxicity [see Clinical Pharmacology (12.3)]. Therefore, consider increased monitoring for local anesthetic systemic toxicity in patients with moderate to severe hepatic disease.
8.7 Renal Impairment
Bupivacaine is known to be substantially excreted by the kidney, and the risk of adverse reactions to EXPAREL may be greater in patients with renal impairment than in patients with normal renal function. Therefore, in patients with renal impairment, increase monitoring for EXPAREL-associated adverse reactions [see Adverse Reactions (6)].
- Study Peds-1 was a multicenter, randomized, open-label, two-part study (NCT03682302) to evaluate the PK and safety of EXPAREL for local infiltration in pediatric patients aged 6 to less than 17 years who were undergoing spine or cardiac surgery (postsurgically, patients were administered opioid rescue medication according to the study site's standard of care).
-
10 OVERDOSAGE
Clinical Presentation
Acute emergencies from local anesthetics are generally related to high plasma concentrations encountered during therapeutic use of local anesthetics or to unintended intravascular injection of local anesthetic solution [See Warnings and Precautions (5) and Adverse Reactions (6)].
Signs and symptoms of overdose include CNS symptoms (perioral paresthesia, dizziness, dysarthria, confusion, mental obtundation, sensory and visual disturbances, and eventually convulsions) and cardiovascular effects (that range from hypertension and tachycardia to myocardial depression, hypotension, bradycardia, and asystole).
Plasma levels of bupivacaine associated with toxicity can vary. Although concentrations of 2,500 to 4,000 ng/mL have been reported to elicit early subjective CNS symptoms of bupivacaine toxicity, symptoms of toxicity have been reported at levels as low as 800 ng/mL.
Management of Local Anesthetic Overdose
At the first sign of change, oxygen should be administered.
The first step in the management of convulsions, as well as underventilation or apnea, consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation with oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support, and if the status of the circulation permits, small increments of an ultra-short acting barbiturate (such as thiopental or thiamylal) or a benzodiazepine (such as diazepam) may be administered intravenously. The clinician should be familiar, prior to the use of anesthetics, with these anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor dictated by the clinical situation (such as ephedrine to enhance myocardial contractile force).
If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias, and cardiac arrest. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated, after initial administration of oxygen by mask, if difficulty is encountered in the maintenance of a patent airway or if prolonged ventilatory support (assisted or controlled) is indicated.
-
11 DESCRIPTION
EXPAREL (bupivacaine liposome injectable suspension) is a sterile, non-pyrogenic white to off-white preservative-free aqueous suspension consisting of multivesicular liposomes containing bupivacaine. Bupivacaine is present at a concentration of 13.3 mg/mL. After injection of EXPAREL, bupivacaine is released from the multivesicular liposomes. EXPAREL is for infiltration or perineural use.
Active Ingredient
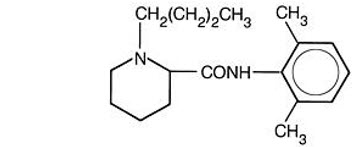
Bupivacaine is related chemically and pharmacologically to the amide-type local anesthetics. It is a homologue of mepivacaine and is related chemically to lidocaine. All three of these anesthetics contain an amide linkage between the aromatic nucleus and the amino, or piperidine group. They differ in this respect from the procaine-type local anesthetics, which have an ester linkage. Chemically, bupivacaine is 1-butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide with a molecular weight of 288.4. Bupivacaine has the following structural formula:
EXPAREL
The median diameter of the liposome particles in EXPAREL ranges from 24 to 31 μm. The liposomes are suspended in a 0.9% Sodium Chloride Injection. Each vial contains bupivacaine at a nominal concentration of 13.3 mg/mL. Inactive ingredients and their nominal concentrations are: cholesterol, 4.7 mg/mL; 1, 2-dipalmitoyl-sn-glycero-3 phospho-rac-(1-glycerol) sodium salt (DPPG), 0.9 mg/mL; tricaprylin, 2.0 mg/mL; 1, 2-dierucoylphosphatidylcholine (DEPC), 8.2 mg/mL; phosphoric acid to adjust pH; and sodium chloride to adjust tonicity. The pH of EXPAREL is in the range of 5.8 to 7.4.
Bupivacaine in EXPAREL has different functional properties relative to those of the unencapsulated or nonlipid-associated bupivacaine products. Bupivacaine that is released from EXPAREL has a different pharmacokinetic and systemic profile relative to other bupivacaine products. In addition, the nominal weight percent concentration of bupivacaine in EXPAREL is based on bupivacaine free base rather than bupivacaine HCl (100 mg of bupivacaine HCl contains 88.6 mg of bupivacaine free base) [see Dosage and Administration (2.1)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Local anesthetics block the generation and the conduction of nerve impulses presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination, and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
12.2 Pharmacodynamics
Systemic absorption of local anesthetics produces effects on the cardiovascular and central nervous systems. At blood concentrations achieved with normal therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations depress cardiac conductivity and excitability, which may lead to atrioventricular block, ventricular arrhythmias, and cardiac arrest, sometimes resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. Clinical reports and animal research suggest that these cardiovascular changes are more likely to occur after accidental intravascular injection of bupivacaine.
Following systemic absorption, local anesthetics can produce central nervous system stimulation, depression, or both. Apparent central stimulation is manifested as restlessness, tremors, and shivering progressing to convulsions, followed by depression and coma progressing ultimately to respiratory arrest. However, the local anesthetics have a primary depressant effect on the medulla and on higher centers. The depressed stage may occur without a prior excited state.
12.3 Pharmacokinetics
After administration of EXPAREL, the systemic plasma levels of bupivacaine were observed for 96 hours after local infiltration, 120 hours after interscalene brachial plexus nerve block, 168 hours after sciatic nerve block in the popliteal fossa, and 168 hours after adductor canal block [see Warnings and Precautions (5.2)]. In general, peripheral nerve blocks have shown systemic plasma levels of bupivacaine for extended duration when compared to local infiltration. Systemic plasma levels of bupivacaine following administration of EXPAREL are not correlated with local efficacy.
Absorption
The rate of systemic absorption of bupivacaine is dependent upon the total dose of EXPAREL administered, the route of administration, and the vascularity of the administration site.
Pharmacokinetic parameters of EXPAREL after local infiltration, and following an interscalene brachial plexus nerve block, sciatic nerve block in the popliteal fossa, and adductor canal block were evaluated following surgical procedures. Descriptive statistics of pharmacokinetic parameters of representative EXPAREL doses in each study are provided in Table 5 for adult patients after administration of single doses of EXPAREL via local infiltration; Table 6 for adult patients after administration of single doses of EXPAREL via nerve block; and in Table 7 for pediatric patients aged 6 to less than 17 years old after administration of single doses of EXPAREL via local infiltration.
Table 5: Summary of Pharmacokinetic Parameters for Bupivacaine after Administration of Single Doses of EXPAREL via Local Infiltration in Adult Patients Parameters* Bunionectomy†
106 mg
(8 mL)Hemorrhoidectomy‡
266 mg
(20 mL)Spine Surgery§
266 mg
(20 mL)Cardiothoracic Surgery¶
266 mg
(20 mL)(N=26) (N=25) (N=11) (N=5) NE: Not evaluated - *
- Arithmetic mean (standard deviation) except Tmax where it is median (minimum, maximum).
- †
- Patients undergoing bunionectomy (Study 1)
- ‡
- Patients undergoing hemorrhoidectomy (Study 2)
- §
- Patients undergoing open posterior spinal fusion or reconstructive surgery
- ¶
- Patients undergoing posterolateral thoracotomy
- #
- AUC0-last, 0-72h;
- Þ
- AUC0-last, 0-96h
Cmax (ng/mL) 166 (93) 867 (353) 513 (268) 445 (120) Tmax (h) 2 (0.5, 24) 0.5 (0.25, 36) 0.6 (0.2, 37) 0.6 (0.6, 36) AUC0-40h
(h × ng/mL)NE NE 13035 (8782) 9867 (1332) AUC(0-last)
(h × ng/mL)5864 (2038) # 16867 (7868) # 17214 (11621) Þ 14277 (3449) Þ AUC(inf)
(h × ng/mL)7105 (2283) 18289 (7569) 17917 (12187) 15768 (4530) t½ (h) 34 (17) 24 (39) 9 (2) 14 (6) Table 6: Summary of Pharmacokinetic Parameters for Bupivacaine after Administration of Single Doses of EXPAREL via Nerve Block in Adult Patients (Studies 3, 4 and 5) Parameters* Interscalene Brachial Plexus Nerve Block† Sciatic Nerve Block in the Popliteal Fossa‡ Adductor Canal Block§ EXPAREL
133 mg (10 mL)EXPAREL
133 mg (10 mL) + BUP as Mayo field block (100 mg¶)
(Total dose: 221.6 mg bupivacaine)EXPAREL
133 mg (10 mL) + BUP 50 mg + BUP as IPACK (37.5 mg) + BUP (up to 15 mg) as spinal anesthesia
(Total dose: 224 mg bupivacaine)(N = 12) (N = 21) (N = 24) BUP: Bupivacaine HCl.
IPACK: Infiltration between the popliteal artery and capsule of the knee
NE: Not evaluated- *
- Arithmetic mean (standard deviation) except Tmax where it is median (minimum, maximum).
- †
- Patients undergoing total shoulder arthroplasty (Study 3)
- ‡
- Patients undergoing bunionectomy (Study 4)
- §
- Patients undergoing total knee arthroplasty (Study 5)
- ¶
- 100 mg bupivacaine HCl contains 88.6 mg of bupivacaine free base.
- #
- AUC0-last, 0-120h ;
- Þ
- AUC0-last, 0-168h
Cmax (ng/mL) 207 (137) 382 (241) 495 (165) Tmax (h) 48 (3, 74) 8.1 (1.7, 12) 0.7 (0.4, 72) AUC(0-last) (h × ng/mL) 11484 (8615)# 16005 (6740) Þ 25039 (11921) Þ AUC(inf) (h × ng/mL) 11590 (8603) 17004 (7206) 25109 (11918) t½ (h) 11 (5) 28 (14) 11 (3) Table 7: Summary of Pharmacokinetic Parameters for Bupivacaine after Administration of Single Doses of EXPAREL via Local Infiltration in Pediatric Patients Aged 6 to Less Than 17 Years Old (Studies Peds-1 and Peds-2) Parameters* Spine Surgery Cardiac Surgery EXPAREL
4 mg/kg
(Maximum 266 mg)EXPAREL
4 mg/kg
(Maximum 266 mg)6 to <17 years
(N = 17)6 to <12 years
(N = 21)NR1 = Not reported, since the last sampling time point varies among different patients.
NR2= Not reported, since the terminal elimination phase was not adequately characterized in sufficient number of patients.Cmax (ng/mL) 353 (125) 447 (243) Tmax (h) 1.2 (0.3-26) 23 (0.2, 55) AUC(0- 40 h) (h × ng/mL) 8782 (2834) 11286 (4791) AUC(0-last) (h × ng/mL) NR1 16776 (7936)† AUC(inf) (h × ng/mL) NR2 NR2 t½ (h) NR2 NR2 Distribution
After bupivacaine has been released from EXPAREL and is absorbed systemically, bupivacaine distribution is expected to be the same as for any bupivacaine HCl solution formulation.
Local anesthetics including bupivacaine are distributed to some extent to all body tissues, with high concentrations found in highly perfused organs such as the liver, lungs, heart, and brain.
Local anesthetics including bupivacaine appear to cross the placenta by passive diffusion. The rate and degree of diffusion is governed by (1) the degree of plasma protein binding, (2) the degree of ionization, and (3) the degree of lipid solubility. Fetal/maternal ratios of local anesthetics appear to be inversely related to the degree of plasma protein binding, because only the free, unbound drug is available for placental transfer. Bupivacaine with a high protein binding capacity (95%) has a low fetal/maternal ratio (0.2 to 0.4). The extent of placental transfer is also determined by the degree of ionization and lipid solubility of the drug. Lipid soluble, non-ionized drugs such as bupivacaine readily enter the fetal blood from the maternal circulation.
Elimination
Metabolism
Amide-type local anesthetics, such as bupivacaine, are metabolized primarily in the liver via conjugation with glucuronic acid. Pipecoloxylidide (PPX) is the major metabolite of bupivacaine; approximately 5% of bupivacaine is converted to PPX. Elimination of drug depends largely upon the availability of plasma protein binding sites in the circulation to carry it to the liver where it is metabolized.
Various pharmacokinetic parameters of the local anesthetics can be significantly altered by the presence of hepatic disease. Patients with hepatic disease, especially those with severe hepatic disease, may be more susceptible to the potential toxicities of the amide-type local anesthetics.
Excretion
After bupivacaine has been released from EXPAREL and is absorbed systemically, bupivacaine excretion is expected to be the same as for other bupivacaine formulations.
The kidney is the main excretory organ for most local anesthetics and their metabolites. Only 6% of bupivacaine is excreted unchanged in the urine.
Urinary excretion is affected by urinary perfusion and factors affecting urinary pH. Acidifying the urine hastens the renal elimination of local anesthetics. Various pharmacokinetic parameters of the local anesthetics can be significantly altered by the presence of renal disease, factors affecting urinary pH, and renal blood flow.
Specific Populations
Hepatic Impairment
Because amide-type local anesthetics, such as bupivacaine, are metabolized by the liver, the effects of decreased hepatic function on bupivacaine pharmacokinetics following administration of EXPAREL were studied in patients with moderate hepatic impairment. Consistent with the hepatic clearance of bupivacaine, mean plasma concentrations were higher in patients with moderate hepatic impairment than in the healthy control volunteers with approximately 1.5- and 1.6-fold increases in the mean values for Cmax and the area under the curve (AUC), respectively. [see Warnings and Precautions (5.1) and Use in Specific Populations (8.6)].
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies in Adult Patients
In five multicenter, randomized, double-blinded, placebo-controlled clinical studies in adults, the efficacy of EXPAREL was established to produce postsurgical:
- Local analgesia via infiltration: One study evaluated the use of EXPAREL in patients undergoing bunionectomy (Study 1); the other study evaluated the use of EXPAREL in patients undergoing hemorrhoidectomy (Study 2) [see Clinical Studies (14.2)].
- Regional analgesia via perineural use: one study (Study 3) evaluated the use of EXPAREL as a brachial plexus nerve block via interscalene or supraclavicular approach in patients undergoing total shoulder arthroplasty (TSA) or rotator cuff repair (RCR) (only two patients had nerve blocks via the supraclavicular approach); one study evaluated the use of EXPAREL as a sciatic nerve block in the popliteal fossa in patients undergoing bunionectomy (Study 4); and one study evaluated the use of EXPAREL as an adductor canal block in patients undergoing total knee arthroplasty (TKA) (Study 5) [see Clinical Studies (14.3)].
Four additional studies (Studies 6, 7, 8, and 9) [see Clinical Studies (14.4)] did not provide sufficient efficacy and/or safety data to support an indication for the following nerve blocks: femoral block in patients undergoing total knee arthroplasty, intercostal nerve block in patients undergoing posterolateral thoracotomy, and combined sciatic (in popliteal fossa) and saphenous (in adductor canal) nerve block [see Indications and Usage (1)].
14.2 Studies in Adults to Produce Postsurgical Local Analgesia
Study 1: Infiltration for Bunionectomy in Adults
A multicenter, randomized, double-blind, placebo-controlled, parallel-group clinical trial (NCT00890682) (Study 1) evaluated the safety and efficacy of 106 mg (8 mL) EXPAREL in 193 adult patients undergoing bunionectomy. The mean age was 43 years (range 18 to 72).
Study medication was administered directly into the site at the conclusion of the surgery, prior to closure. There was an infiltration of placebo or 7 mL of EXPAREL into the tissues surrounding the osteotomy and 1 mL into the subcutaneous tissue.
Pain intensity was rated by the patients on a 0 to 10 numeric rating scale (NRS) out to 72 hours. Postoperatively, patients were allowed rescue medication (5 mg oxycodone/325 mg acetaminophen orally every 4 to 6 hours as needed) or, if that was insufficient within the first 24 hours, ketorolac (15 to 30 mg IV). The primary outcome measure was the area under the curve (AUC) of the NRS pain intensity scores (cumulative pain scores) collected over the first 24-hour period. There was a significant treatment effect for EXPAREL compared to placebo. EXPAREL demonstrated a significant reduction in pain intensity compared to placebo for up to 24 hours. There was no significant difference in the amount of morphine equivalents used through 72 hours post-surgery, 43 mg versus 42 mg for placebo and EXPAREL, respectively. In addition, there was not a significant difference in the percentage of patients that used ketorolac, 43% versus 31% for placebo and EXPAREL, respectively.
Study 2: Infiltration for Hemorrhoidectomy in Adults
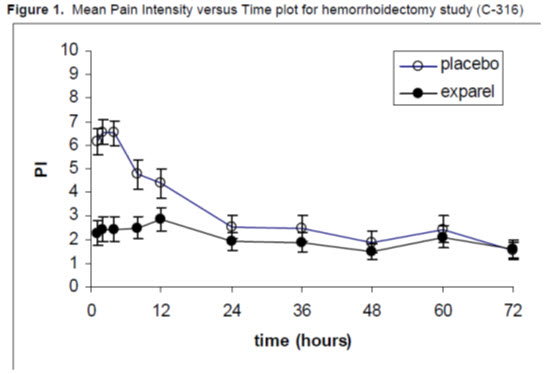
A multicenter, randomized, double-blind, placebo-controlled, parallel-group clinical trial (NCT00890721) (Study 2) evaluated the safety and efficacy of 266 mg (20 mL) EXPAREL in 189 adult patients undergoing hemorrhoidectomy. The mean age was 48 years (range 18 to 86).
EXPAREL or placebo were administered directly into the site (greater than or equal to 3 cm) at the conclusion of the surgery. Dilution of 20 mL of EXPAREL with 10 mL of saline, for a total of 30 mL, was divided into six 5-mL aliquots. A field block was performed by visualizing the anal sphincter as a clock face and slowly infiltrating one aliquot to each of the even numbers.
Pain intensity was rated by the patients on a 0 to 10 NRS at multiple time points up to 72 hours. Postoperatively, patients were allowed rescue medication (morphine sulfate 10 mg intramuscular every 4 hours as needed).
The primary outcome measure was the AUC of the NRS pain intensity scores (cumulative pain scores) collected over the first 72-hour period.
There was a significant treatment effect for EXPAREL compared to placebo. See Figure 1 for the mean pain intensity over time for the EXPAREL and placebo treatment groups for the 72-hour efficacy period.
There were statistically significant, but small differences in the amount of opioid rescue analgesia used across the treatment groups, the clinical benefit of which has not been established. The median time to rescue analgesic use was 15 hours for patients treated with EXPAREL and one hour for patients treated with placebo. Twenty-eight percent of patients treated with EXPAREL required no rescue medication at 72 hours compared to 10% treated with placebo. For those patients who did require rescue medication, the mean amount of morphine sulfate intramuscular injections used over 72 hours was 22 mg for patients treated with EXPAREL and 29 mg for patients treated with placebo.
14.3 Nerve Block Studies in Adults to Produce Postsurgical Regional Analgesia
Study 3: Interscalene Brachial Plexus Nerve Block for Total Shoulder Arthroplasty or Rotator Cuff Repair in Adults
A multicenter, randomized, double-blind, placebo-controlled study (NCT02713230) (Study 3) was conducted in 156 adult patients undergoing primary unilateral total shoulder arthroplasty or rotator cuff repair with general anesthesia. The mean age was 61 years (range 33 to 80). Prior to the surgical procedure, patients received 133 mg (10 mL) of EXPAREL expanded with normal saline to 20 mL or placebo as a brachial plexus nerve block (perineural use) via interscalene or supraclavicular approach with ultrasound guidance. Only two patients received nerve block with EXPAREL by supraclavicular approach. Postsurgically, patients were administered acetaminophen/paracetamol up to 1,000 mg orally or intravenously every 8 hours unless contraindicated. Patients were allowed opioid rescue medication administered initially as oral immediate-release oxycodone (initiating at 5-10 mg every 4 hours or as needed). If a patient could not tolerate oral medication, intravenous morphine (2.5-5 mg) or hydromorphone (0.5-1 mg) could be administered every 4 hours or as needed.
In Study 3, there was a statistically significant treatment effect for EXPAREL compared to placebo in cumulative pain scores through 48 hours as measured by the AUC of the visual analog scale (VAS) pain intensity scores. There were statistically significant, but small differences in the amount of opioid consumption through 48 hours, the clinical benefit of which has not been demonstrated. For those patients who required rescue medication, the mean amount of morphine-equivalent opioid rescue used over 48 hours was 12 mg for EXPAREL-treated patients and 54 mg for placebo-treated patients and 23 mg with EXPAREL-treated patients vs. 70 mg for placebo-treated patients over 72 hours.
Although at 48 hours, 9 patients (13%) in the EXPAREL group remained opioid-free compared to 1 patient (1%) in the placebo group, a difference which was statistically significant, at 72 hours, there were 4 (6%) patients in the EXPAREL group who remained opioid-free compared to 1 (1%) patient in the placebo group, a difference that is not statistically significant.
Study 4: Sciatic Nerve Block in the Popliteal Fossa for Bunionectomy in Adults
A multicenter, randomized, double-blind, active controlled study (NCT05157841) (Study 4) was conducted in 185 adult patients undergoing bunionectomy. The mean age was 49 years (range 18 to 76).
Prior to the surgical procedure, patients received 133 mg of EXPAREL mixed with 20 mL saline OR 50 mg (20 mL) of 0.25% bupivacaine HCl mixed with 10 mL saline via sciatic nerve block (perineural use) in the popliteal fossa. All patients received 100 mg (20 mL) of 0.5% immediate-release bupivacaine HCl as a Mayo field block after study drug administration (i.e., in the operating room immediately prior to surgical incision). All patients also received a dose of 1,000 mg of intravenous (IV) acetaminophen at the time of surgical incision. IV fentanyl was allowed for intraoperative pain control (not exceeding 1 mcg/kg unless deemed medically necessary).
Postsurgically, all patients received one post-operative dose of 1,000 mg IV acetaminophen, administered approximately 8 hours after the first dose (approximately 8 hours after incision). Patients were administered pain medications on an as needed basis; opioids were not given on a pre-determined schedule. Patients were allowed opioid rescue medication administered initially as oral immediate-release oxycodone (initiating at 5 mg and increasing the dose to 10 mg if initial opioid dose was insufficient). If a patient could not tolerate oral medication or the oral oxycodone pain relief was insufficient, IV morphine (initiated at 2 mg) or hydromorphone (initiated at 0.2 mg) could be administered. No NSAIDs or other opioids including tramadol were allowed for the breakthrough pain management and no acetaminophen (other than the scheduled IV acetaminophen) was used for breakthrough pain.
In Study 4, there was a statistically significant treatment effect for EXPAREL (with 0.5% immediate-release bupivacaine HCl as a Mayo field block) compared to bupivacaine HCl (with 0.5% immediate-release bupivacaine HCl as a Mayo field block) in cumulative pain scores through 96 hours as measured by AUC of the NRS pain intensity scores. There were statistically significant differences in the total amount of postsurgical opioid medication used through 96 hours, the clinical benefit of which has not been demonstrated. For those patients who required postsurgical opioid medication, the mean amount of morphine-equivalent opioid rescue used over 96 hours was 18 mg for patients treated with EXPAREL + Mayo field block and 45 mg for patients treated with bupivacaine HCl + Mayo field block. There was also a statistically significant difference in the percentage of opioid-free patients through 96 hours compared with bupivacaine HCl (24% in the EXPAREL + Mayo field block vs. 6% in the bupivacaine HCl + Mayo field block).
See Figure 2 for the LS mean pain intensity scores after administration of either 133 mg of EXPAREL or 50 mg of 0.25% bupivacaine HCl.
Figure 2. NRS Pain Intensity After Administration of Study Drug by Sciatic Nerve Block in the Popliteal Fossa.
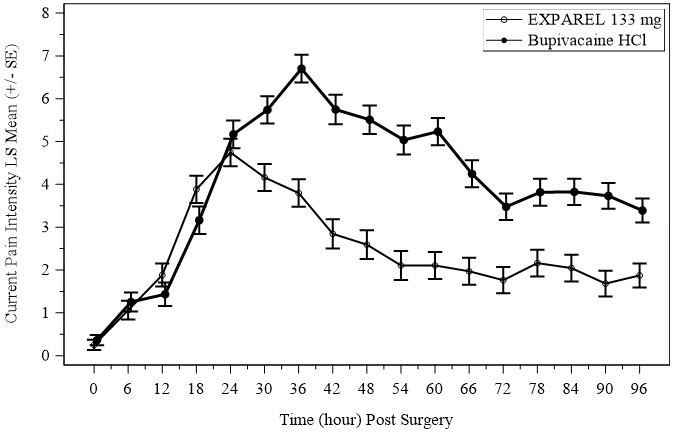
Study 5: Adductor Canal Block for Total Knee Arthroplasty in Adults
A multicenter, randomized, double-blind, active controlled study (NCT05139030) (Study 5) was conducted in 166 adult patients undergoing primary unilateral total knee arthroplasty. The mean age was 62 years (range 37 to 83).
Prior to the surgical procedure, patients received (via adductor canal block) (perineural use):
- 133 mg (10 mL) of 1.3% EXPAREL admixed with 50 mg (10 mL) of 0.5% bupivacaine HCl or
- 50 mg (10 mL) of 0.5% bupivacaine HCl mixed with 10 mL normal saline.
All patients also received 37.5 mg (15 mL) of 0.25% immediate-release bupivacaine HCl as an infiltration between the popliteal artery and capsule of the knee (IPACK) block immediately following study drug administration. All patients received spinal anesthesia immediately prior to surgery with 0.5% bupivacaine HCl (up to 15 mg). All patients also received a dose of 1,000 mg of intravenous (IV) acetaminophen at the time of surgical incision. IV fentanyl was allowed for intraoperative pain control (not exceeding 1 ug/kg unless deemed medically necessary).
Postsurgically, all patients received one post-operative dose of 1,000 mg IV acetaminophen, administered approximately 8 hours after the first dose (approximately 8 hours after incision). Patients were administered pain medications on an as needed basis for breakthrough pain; opioids were not given on a pre-determined schedule. Patients were allowed opioid rescue medication administered initially as oral immediate-release oxycodone (initiating at 5 mg and increasing the dose to 10 mg if initial opioid dose was insufficient). If a patient could not tolerate oral medication or the oral oxycodone pain relief was insufficient, IV morphine (initiated at 2 mg) or hydromorphone (initiated at 0.2 mg) could be administered. No NSAIDs or other opioids including tramadol were allowed for the breakthrough pain management and no acetaminophen (other than the scheduled IV acetaminophen) was used for breakthrough pain.
In Study 5, there was a statistically significant treatment effect for EXPAREL + IPACK block compared to bupivacaine HCl + IPACK in cumulative pain scores through 96 hours as measured by AUC of the NRS pain intensity scores. There were statistically significant differences in the total amount of postsurgical opioid medication used through 96 hours, the clinical benefit of which has not been demonstrated. For those patients who required postsurgical opioid medication, the mean amount of morphine-equivalent opioid rescue used over 96 hours was 102 mg for patients treated with EXPAREL + IPACK and 133 mg for patients treated with bupivacaine HCl+ IPACK. The median time to first postsurgical use was 4.2 hours for patients treated with EXPAREL + IPACK and 3.6 hours for patients treated with bupivacaine HCl + IPACK.
See Figure 3 for LS mean pain intensity scores after administration of either 133 mg of 1.3% EXPAREL admixed with 50 mg) of 0.5% bupivacaine HCl or 50 mg of 0.5% bupivacaine HCl.
Figure 3. NRS Pain Intensity After Administration of Study Drug by Adductor Canal Block
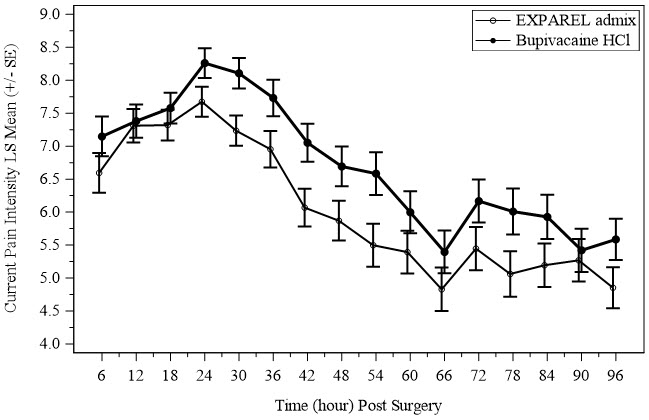
14.4 Studies That Do Not Support an Indication for Certain Nerve Blocks in Adults
Studies 6 and 7: Inadequate Demonstration of Efficacy and Safety in Femoral Nerve Block in Total Knee Arthroplasty
EXPAREL was administered via a femoral nerve block in two placebo-controlled studies in adults (Studies 6 and 7). The results of these studies did not support a femoral nerve block indication due to inadequate safety data (Study 6) and/or due to inadequate efficacy findings (Study 7). In addition, patient falls were reported only in the EXPAREL treatment groups, and none was reported in placebo groups.
Study 6
Study 6, a multicenter, randomized, double-blind, parallel-group, placebo-controlled study (NCT01683071), was conducted in 196 adult patients undergoing primary unilateral total knee arthroplasty (TKA) under general or spinal anesthesia. The mean age was 65 years (range 42 to 88). Prior to the surgical procedure, 266 mg (20 mL) of EXPAREL or placebo was administered as a femoral nerve block with ultrasound guidance. Postsurgically, patients were allowed opioid rescue medication administered initially by intravenous injection of hydromorphone and subsequently by a patient-controlled analgesia (PCA) pump containing morphine or hydromorphone only. Once patients were tolerating oral medication, oral immediate-release oxycodone was administered on an as-needed basis (but not more than 10 mg every 4 hours) or, if that was insufficient, a third rescue of 1.25 mg/mL of 0.125% bupivacaine HCl was administered at a rate of 8 mL per hour via the previously placed femoral nerve catheter.
In this study, there was a statistically significant treatment effect for EXPAREL compared to placebo in cumulative pain scores through 72 hours as measured by the AUC of the NRS pain (at rest) intensity scores.
There was a statistically significant, although small decrease in opioid consumption for the EXPAREL treatment group compared to the placebo group, the clinical benefit of which has not been established. All patients in both the EXPAREL and placebo treatment groups required opioid rescue medication during the first 72 hours. The mean amount of opioid rescue used over 72 hours was 76 mg for patients treated with EXPAREL and 103 mg for patients treated with placebo.
The study was inadequate to fully characterize the safety of EXPAREL when used for femoral nerve block due to patient falls, which occurred only in the EXPAREL-treated patients and not in the placebo-treated patients.
Study 7
Study 7, a multicenter, randomized, double-blind, parallel-group, placebo-controlled study (NCT02713178), was conducted in 230 adult patients undergoing primary unilateral total knee arthroplasty (TKA) under general or spinal anesthesia. The mean age was 65 years (range 39 to 89). Prior to the surgical procedure, either 266 mg (20 mL) of EXPAREL or 133 mg (10 mL) of EXPAREL plus 10 mL of normal saline or placebo was administered as a femoral nerve block with ultrasound guidance. In addition to study drug, 8 mL of 0.5% bupivacaine HCl diluted with 8 mL of normal saline was administered by the surgeon as a periarticular infiltration to the posterior capsule (8 mL each behind the medial and lateral condyles) before placement of the prosthesis. Postsurgically, patients were allowed opioid rescue medication consisting of oral immediate-release oxycodone (initiated at 5 to 10 mg every 4 hours or as needed). If a patient could not tolerate oral medication, IV morphine (2.5 to 5 mg) or hydromorphone (0.5 to 1 mg) was permitted every 4 hours or as needed. Patient-controlled analgesia was not permitted. No other analgesic agents, including NSAIDs, were permitted through 108 hours. However, to reflect the current standard of care of postsurgical multimodal therapy, all patients received cyclobenzaprine (a single dose of 10 mg orally or as needed) and acetaminophen/paracetamol (up to 1,000 mg orally or IV every 8 hours for a maximum total daily dose of 3000 mg) postsurgically.
In this study there were no statistically significant treatment effects for the EXPAREL group compared to the placebo group in cumulative pain intensity scores or total opioid consumption. All patients in the EXPAREL and placebo treatment groups required opioid rescue medication over 72 hours. The median Tmax of bupivacaine observed in this study was 72 hours with a range of 2.5 to 108 hours. Similar to Study 6, patient falls only occurred in the EXPAREL-treated patients and not the placebo-treated patients.
Study 8: Lack of Efficacy in Intercostal Nerve Block for Posterolateral Thoracotomy
A multicenter, randomized, double-blind, placebo-controlled study was conducted in 191 adult patients undergoing posterolateral thoracotomy under general anesthesia (NCT01802411) (Study 8). The mean age was 58 years (range 18 to 82).
After the surgical procedure was completed but prior to the surgical site closure, 266 mg (20 mL) of EXPAREL was administered by the surgeon as an intercostal nerve block divided into three equal doses in three syringes of approximately 88 mg in 6.6 mL volume per nerve, and administered to each of three nerve segments (index nerve, nerve above, and nerve below). Postsurgically, patients were allowed opioid rescue medication administered initially by intravenous fentanyl 100 mcg, which was to be administered once via bolus only. For the US sites, the second rescue medication was to be PCA-administered morphine or hydromorphone. For the European sites, the second rescue medication was to be intramuscular administered morphine up to 10 mg every 4 hours. At all sites, once a patient was tolerating oral medication, oral immediate-release oxycodone was administered (but not more than 10 mg every 4 hours). Patients who did not achieve adequate pain relief with this regimen were to be withdrawn from the study and followed for safety only.
In this study there were no statistically significant treatment effects for EXPAREL 266 mg compared to placebo in cumulative pain intensity scores or total opioid consumption. Four percent of patients treated with EXPAREL required no rescue medication at 72 hours compared to 1% treated with placebo. For those patients who did require rescue medication, the mean amount of opioid rescue used over 72 hours was 71 mg for patients treated with EXPAREL and 71 mg for patients treated with placebo. The median Tmax of bupivacaine observed in this study was 1 hour with a range of 0.5 hours to 50 hours.
Study 9: Lack of Efficacy in Combined Sciatic (in popliteal fossa) and Saphenous (in adductor canal) Nerve Block Study in Lower Extremity Surgeries
A multicenter, randomized, double-blind, active-controlled trial (NCT04518462) (Study 9) was conducted in 119 adult patients who underwent foot and ankle procedures (bunionectomy, first metatarsophalangeal fusion, forefoot & hindfoot fusion, total ankle arthroplasty). The mean age was 48 years (range 20 to 72). Prior to the surgical procedure, patients received one of the following three treatments under ultrasound guidance as a sciatic nerve block in the popliteal fossa and a saphenous nerve block in the adductor canal:
- 266 mg of EXPAREL (20 mL) admixed with 20 mL of 0.9% Sodium Chloride Injection (referred to as normal saline (NS)),
- 266 mg of EXPAREL (20 mL) admixed with 50 mg of 0.25% bupivacaine HCl (20 mL), or
- 100 mg of 0.25% bupivacaine (40 mL).
The total volume (40 mL) was divided evenly between the two blocks. All patients received general anesthesia or nonopioid sedation (except fentanyl); were allowed medication for intraoperative nausea/vomiting prevention; and were allowed medication for breakthrough pain postsurgery which followed a step-wise approach starting with acetaminophen or a nonsteroidal anti-inflammatory drug (not exceeding the maximum dose), and, if needed escalating to an initial 5 mg dose of oxycodone, then to a 10 mg dose of oxycodone, and finally to intravenous morphine (initiated at 2 mg) or intravenous hydromorphone (initiated at 0.2 mg).
In Study 9 there was no significant difference in the primary endpoint [least square mean (LSM) area under the curve (AUC) numerical rating scale (NRS) pain score from 0 to 96 hours after surgery] between EXPAREL + NS and the 0.25% bupivacaine HCl treatment groups. Cumulative opioid consumption differences between groups were nonsignificant 0-96 hours after surgery. The LSM amount of opioids received over 96 hours was 21 mg in EXPAREL group and 20 mg in bupivacaine HCl group.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
EXPAREL (bupivacaine liposome injectable suspension) is a white to off-white milky aqueous suspension that is available in the following single-dose vials.
- 1.3% (266 mg/20 mL) (13.3 mg/mL) single-dose vial, (NDC 65250-266-20) packaged in cartons of 10 (NDC 65250-266-09) and cartons of 4 (NDC 65250-266-04)
- 1.3% (133 mg/10 mL) (13.3 mg/mL) single-dose vial, (NDC 65250-133-10) packaged in cartons of 10 (NDC-65250-133-09) and cartons of 4 (NDC 65250-133-04)
Storage
Store EXPAREL vials refrigerated between 2°C to 8°C (36°F to 46°F). EXPAREL may be held at a controlled room temperature of 20°C to 25°C (68°F to 77°F) for up to 30 days in sealed, intact (unopened) vials. Do not re-refrigerate vials.
Do not freeze or expose EXPAREL to high temperatures (greater than 40°C or 104°F) for an extended period. Do not administer EXPAREL if it is suspected of having been frozen or exposed to high temperatures. Do not use the vial if the stopper is bulging.
-
17 PATIENT COUNSELING INFORMATION
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Inform patients in advance that EXPAREL can cause temporary loss of sensation or motor activity that may last for up to 5 days.
-
SPL UNCLASSIFIED SECTION
Manufactured for Pacira Pharmaceuticals, Inc., a wholly owned subsidiary of Pacira BioSciences, Inc.
San Diego, CA 92121 USATrademark of Pacira Pharmaceuticals, Inc.
©2023 Pacira Pharmaceuticals, Inc. All rights reserved.
For additional information call 1-855-RX-EXPAREL (1-855-793-9727) or visit www.EXPAREL.com
- PRINCIPAL DISPLAY PANEL - 133 mg/10 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 133 mg/10 mL Vial Carton - NDC 65250-133-09
NDC 65250-133-09
Contains 10
10 mL VialsEXPAREL®
(bupivacaine liposome injectable suspension)1.3%
133 mg/10 mL (13.3 mg/mL)Rx Only
For infiltration, interscalene brachial plexus nerve block, sciatic nerve block
in the popliteal fossa, and adductor canal blockContents: Each 10 mL vial contains 133 mg of bupivacaine free base.
Usual Dosage: see Prescribing Information. Do not substitute for or with other
formulations containing bupivacaine or bupivacaine HCl. Single dose vial;
discard any unused portion.Do not freeze. Do not use the vial if the stopper is bulging.
-
PRINCIPAL DISPLAY PANEL - 133 mg/10 mL Vial Carton - NDC 65250-133-04
NDC 65250-133-04
Contains 4
10 mL VialsEXPAREL®
(bupivacaine liposome injectable suspension)1.3%
133 mg/10 mL (13.3 mg/mL)Rx Only
For infiltration, interscalene brachial plexus nerve block, sciatic nerve block
in the popliteal fossa, and adductor canal blockContents: Each 10 mL vial contains 133 mg of bupivacaine free base.
Usual Dosage: see Prescribing Information. Do not substitute for or with other
formulations containing bupivacaine or bupivacaine HCl. Single dose vial;
discard any unused portion.Do not freeze. Do not use the vial if the stopper is bulging.
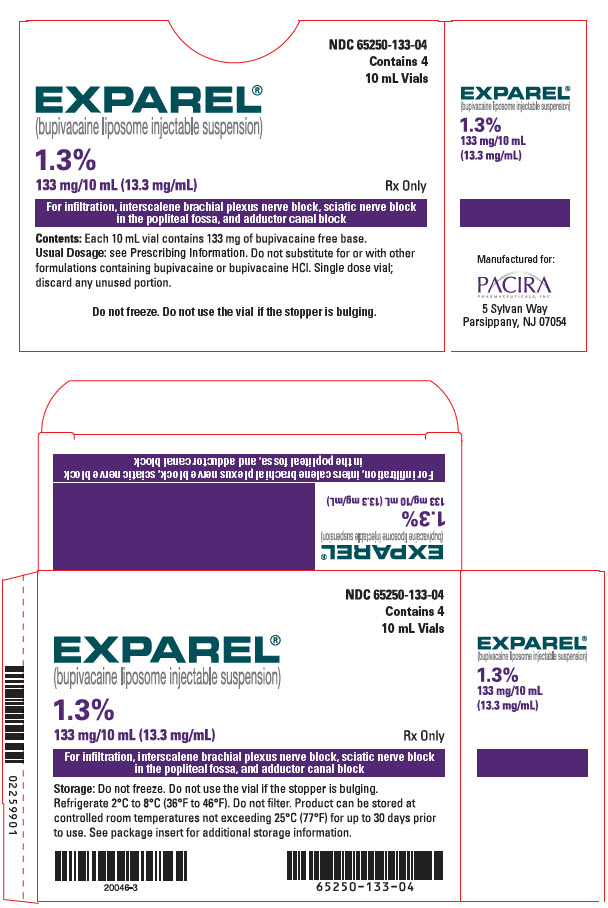
- PRINCIPAL DISPLAY PANEL - 266 mg/20 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 266 mg/20 mL Vial Carton - NDC 65250-266-09
NDC 65250-266-09
Contains 10
20 mL VialsEXPAREL®
(bupivacaine liposome injectable suspension)1.3%
266 mg/20 mL (13.3 mg/mL)Rx Only
For infiltration, interscalene brachial plexus nerve block, sciatic nerve block
in the popliteal fossa, and adductor canal blockContents: Each 20 mL vial contains 266 mg of bupivacaine free base.
Usual Dosage: see Prescribing Information. Do not substitute for or with other
formulations containing bupivacaine or bupivacaine HCl. Single dose vial;
discard any unused portion.Do not freeze. Do not use the vial if the stopper is bulging.

-
PRINCIPAL DISPLAY PANEL - 266 mg/20 mL Vial Carton - NDC 65250-266-04
NDC 65250-266-04
Contains 4
20 mL VialsEXPAREL®
(bupivacaine liposome injectable suspension)1.3%
266 mg/20 mL (13.3 mg/mL)Rx Only
For infiltration, interscalene brachial plexus nerve block, sciatic nerve block
in the popliteal fossa, and adductor canal blockContents: Each 20 mL vial contains 266 mg of bupivacaine free base.
Usual Dosage: see Prescribing Information. Do not substitute for or with other
formulations containing bupivacaine or bupivacaine HCl. Single dose vial;
discard any unused portion.Do not freeze. Do not use the vial if the stopper is bulging.
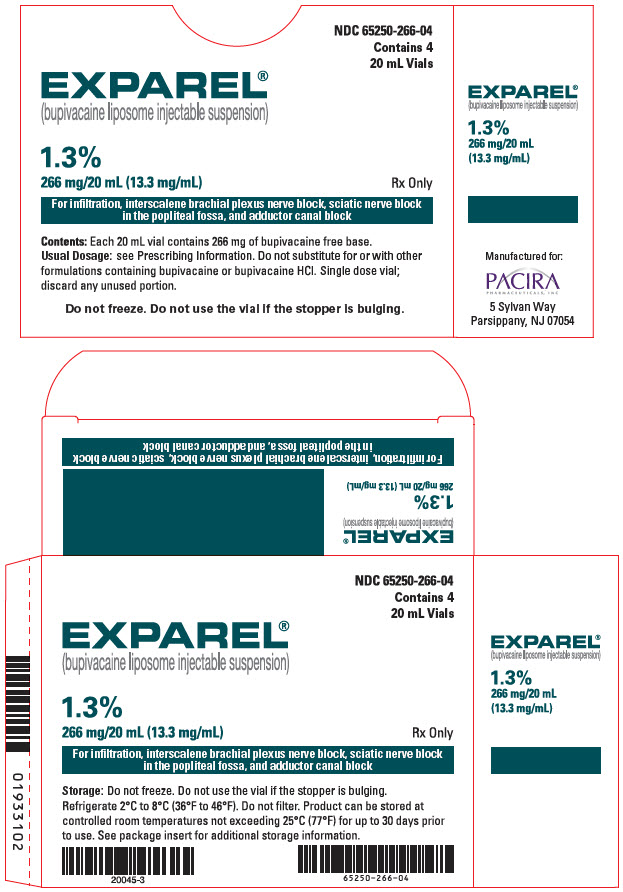
-
INGREDIENTS AND APPEARANCE
EXPAREL
bupivacaine injection, suspension, liposomalProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65250-133 Route of Administration INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE (UNII: Y8335394RO) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE 13.3 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65250-133-09 10 in 1 CARTON 09/02/2016 1 NDC:65250-133-10 10 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:65250-133-04 4 in 1 CARTON 09/02/2016 2 NDC:65250-133-10 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022496 01/01/2016 EXPAREL
bupivacaine injection, suspension, liposomalProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65250-266 Route of Administration INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE (UNII: Y8335394RO) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE 13.3 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65250-266-09 10 in 1 CARTON 03/31/2017 1 NDC:65250-266-20 20 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:65250-266-04 4 in 1 CARTON 09/28/2016 2 NDC:65250-266-20 20 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022496 01/01/2012 Labeler - Pacira Pharmaceuticals, Inc. (783298615) Establishment Name Address ID/FEI Business Operations Pacira Pharmaceuticals, Inc. 117399934 ANALYSIS(65250-133, 65250-266) , MANUFACTURE(65250-133, 65250-266)