SCOPOLAMINE HYDROBROMIDE - scopolamine hydrobromide injection, solution
Fresenius Kabi USA, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Scopolamine Hydrobromide Injection USP
FOR INTRAMUSCULAR, INTRAVENOUS OR SUBCUTANEOUS USE
Protect From Light
Opaque covering needed until contents used
DESCRIPTION
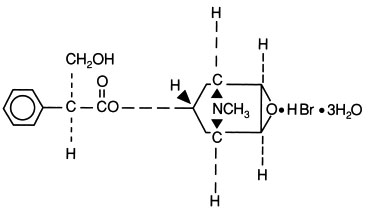
Scopolamine Hydrobromide Injection, USP is a sterile solution of scopolamine hydrobromide (C17H21NO4 • HBr • 3H2O) in Water for Injection. The injection is preserved with methylparaben 0.18% and propylparaben 0.02%. Scopolamine Hydrobromide Injection is intended for intramuscular, intravenous and subcutaneous use. The pH (3.5-6.5) is adjusted with hydrobromic acid if necessary.
The structural formula is:
CLINICAL PHARMACOLOGY
Scopolamine hydrobromide is one of the major antimuscarinic agents that inhibit the action of acetylcholine (ACh) on autonomic effectors innervated by postganglionic cholinergic nerves as well as on smooth muscles that lack cholinergic innervation. It exerts little effects on the actions of ACh at nicotinic receptor sites such as autonomic ganglia. The major action of this antimuscarinic agent is a surmountable antagonism to ACh and other muscarinic agents.
As compared with atropine, scopolamine differs only quantitatively in antimuscarinic actions. Scopolamine has a stronger action on the iris, ciliary body and certain secretory glands such as salivary, bronchial and sweat. Scopolamine, in therapeutic doses, normally causes drowsiness, euphoria, amnesia, fatigue and dreamless sleep with a reduction in rapid-eye-movement sleep. However, the same doses occasionally cause excitement, restlessness, hallucinations or delirium, especially in the presence of severe pain. Scopolamine depresses the EEG arousal response to photostimulation. It is more potent than atropine on the antitremor activity (parkinsonism) in animals induced by surgical lesions. Scopolamine is effective in preventing motion sickness by acting on the maculae of the utricle and saccule.
Scopolamine, although less potent than atropine, has been used frequently in preanesthetic medication for the purpose of inhibiting the secretions of the nose, mouth, pharynx and bronchi and reduces the occurrence of laryngospasm during general anesthesia. Scopolamine is less potent in the decrease of cardiac rate, but not in the changes of blood pressure or cardiac output. Like other antimuscarinic agents, scopolamine has been used widely in the treatment of peptic ulcers and as an antispasmodic agent for G.I. disorders. This is due to the fact that scopolamine reduces salivary secretion, the gastric secretion (both the volume and acid content), and also it inhibits the motor activity of the stomach, duodenum, jejunum, ileum and colon, characterized by a decrease in tone, amplitude and frequency of peristaltic contractions.
INDICATIONS AND USAGE
Scopolamine Hydrobromide Injection, USP is indicated as a sedative and tranquilizing depressant to the central nervous system. In its peripheral actions, scopolamine differs from atropine in that it is a stronger blocking agent for the iris, ciliary body and salivary, bronchial and sweat glands but is weaker in its action on the heart (in which it is incapable of exerting actions in tolerated doses), the intestinal tract and bronchial musculature.
In addition to the usual uses for antimuscarinic drugs, scopolamine is employed for its central depressant actions as a sedative. Frequently it is given as a preanesthetic medicament for both its sedative-tranquilizing and antisecretory actions. It is an effective antiemetic. It is used in maniacal states, in delirium tremens and in obstetrics. As a mydriatic and cycloplegic, it has a somewhat shorter duration (3 to 7 days) and intraocular pressure is affected less markedly than with atropine.
CONTRAINDICATIONS
Scopolamine hydrobromide is contraindicated in patients with narrow-angle glaucoma, since administration of the drug could raise the intraocular pressure to dangerous levels. However, this will not happen for side-angle glaucoma patients. Repeated administration of scopolamine to a patient with chronic lung disease is considered to be potentially hazardous. Patients hypersensitive to belladonna or to barbiturates may be hypersensitive to scopolamine hydrobromide.
WARNINGS
Addiction does not occur, although vomiting, malaise, sweating and salivation have been reported in patients with parkinsonism upon sudden withdrawal of large doses of scopolamine. Scopolamine is one of the most important drugs of the belladonna group from the standpoint of poisoning; infants and young children are especially susceptible to the belladonna alkaloids. Scopolamine is usually stated more toxic than atropine. Idiosyncrasy is more common with scopolamine than with atropine and ordinary therapeutic doses sometimes cause alarming reactions.
PRECAUTIONS
General
If there is mydriasis and photophobia, dark glasses should be worn. Appropriate dosage precautions must be taken with infants, children, persons with mongolism, brain damage, spasticity, or light irides. Elevated intraocular pressure, urinary difficulty and retention and constipation are more probable in elderly persons. Men with prostatic hypertrophy should especially be monitored for urinary function. Because of the tachycardic effects of the drugs, care must be exercised when tachycardia, other tachyarrhythmias, coronary heart disease, congestive heart disease or hyperthyroidism preexist. Persons with hypertension may experience both exaggerated orthostatic hypotension and tachycardia. Similarly, autonomic neuropathy requires caution. Persons with a history of allergies or bronchial asthma will show a higher than normal incidence of hypersensitivity reactions.
Laboratory Tests
Barbiturates may increase Bromosulfonphthalein (BSP) levels; administration is not recommended during the 24 hours preceding the test.
Drug Interactions
Other drugs, such as phenothiazines, tricyclic antidepressants, certain antihistamines, meperidine, etc., which have weak antimuscarinic activity, may considerably intensify the effects of antimuscarinic drugs. Aluminum- and magnesium trisilicate-containing antacids have been shown to decrease the absorption of some antimuscarinic drugs and may possibly do so with all of them.
Pregnancy Category C
Scopolamine hydrobromide can pass the placental barrier; the threat to the fetus in utero is unknown, but use during pregnancy may cause respiratory depression in the neonate and may contribute to neonatal hemorrhage due to reduction in Vitamin K-dependent clotting factors in the neonate.
Scopolamine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
ADVERSE REACTIONS
With nearly all antimuscarinic drugs, dry mouth is the first and dry skin is the second most common side effect. Thirst and difficulty in swallowing occur when the mouth and esophagus become sufficiently dry; chronic dry mouth also fosters dental caries. Suppression of sweating causes reflexive flushing and heat intolerance and can result in heat exhaustion or heat stroke in a hot environment; it also contributes to the hyperthermia seen in intoxication. Mydriasis frequently occurs, especially with scopolamine; photophobia and blurring of vision are consequences of mydriasis. Cycloplegia (which exacerbates blurred vision) occurs approximately concomitantly with mydriasis, but usually higher doses are required. In susceptible persons, especially the elderly, cycloplegia may contribute to an elevation of intraocular pressure. Difficulty in urination and urinary retention may occur. Tachycardia is a common side effect. Constipation, even bowel stasis, may occur.
In the larger therapeutic doses, scopolamine may cause dizziness, restlessness, tremors, fatigue and locomotor difficulties.
OVERDOSAGE
Serious systemic intoxication can occur even from topical ophthalmologic application, especially in children, since both local absorption and nasolacrimal drainage into the gut can deliver considerable amounts to the circulation. In serious intoxication, hyperpyrexia, flushing, nausea, vomiting, drowsiness, disorientation, stupor, hallucinations, leukocytosis, nonallergenic rashes, circulatory or respiratory collapse, even death, in addition to all aforenamed effects, may occur. Children, especially infants and children with mongolism, spastic paralysis or brain damage, are more sensitive than adults to the toxic effects.
If marked excitement is present and more specific treatment is not available, diazepam is most suitable for sedation and for control of convulsions. Large doses should be avoided because the central depressant action may coincide with the depression occurring late in belladonna poisoning. Phenothiazines should not be used because their antimuscuranic action is likely to intensify toxicity and may plunge the patient into coma. Artificial respiration with oxygen may be necessary. Ice bags and alcohol sponges help to reduce fever, especially in children.
DOSAGE AND ADMINISTRATION
Adult
For obstetric amnesia or preoperative sedation, 0.32 to 0.65 mg (320 to 650 mcg).
For sedation or tranquilization, 0.6 mg (600 mcg) 3 or 4 times a day.
Subcutaneous, as an antiemetic, 0.6 to 1 mg.
Pediatric
Age 6 mo. to 3 yr., 0.1 to 0.15 mg (100 to 150 mcg). Age 3 to 6 yr., 0.2 to 0.3 mg (200 to 300 mcg).
Subcutaneous, as antiemetic, 0.006 mg (6 mcg) per kg.
Dosage Equivalents
| 1 mg (1000 mcg)/mL |
||
| 1 mg | (1000 mcg) | 1 mL |
| 0.8 mg | (800 mcg) | 0.8 mL |
| 0.6 mg | (600 mcg) | 0.6 mL |
| 0.5 mg | (500 mcg) | 0.5 mL |
| 0.4 mg | (400 mcg) | 0.4 mL |
| 0.3 mg | (300 mcg) | 0.3 mL |
| 0.2 mg | (200 mcg) | 0.2 mL |
| 0.1 mg | (100 mcg) | 0.1 mL |
| 0.4 mg (400 mcg)/mL |
||
| 0.4 mg | (400 mcg) | 1 mL |
| 0.3 mg | (300 mcg) | 0.75 mL |
| 0.25 mg | (250 mcg) | 0.63 mL |
| 0.2 mg | (200 mcg) | 0.50 mL |
| 0.15 mg | (150 mcg) | 0.38 mL |
Belladonna alkaloids provide a therapeutic effect in about 1 or 2 hours with a duration of about 4 hours.
Geriatric and debilitated patients may respond to the usual doses with excitement, agitation, drowsiness or confusion; lower doses may be required in such patients.
Close supervision is recommended for infants, blondes, mongoloids and children with spastic paralysis or brain damage, since an increased responsiveness to belladonna alkaloids has been reported in these patients and dosage adjustments are often required.
Administration of belladonna alkaloids and barbiturates 30 to 60 minutes before meals is recommended to maximize absorption and, when issued for reducing stomach acid formation, to allow its effect to coincide better with antacid administration following the meal.
Parenteral drug products should be inspected visually for particulate matter prior to administration, whenever solution and container permit.
HOW SUPPLIED
Scopolamine Hydrobromide Injection, USP is supplied as:
| Product
No. | NDC
No. | Strength |
|
| 26801 | 63323-268-01 | 0.4 mg/mL | 1 mL in a 2 mL multiple dose vial, in packages of 25. |
| 27001 | 63323-270-01 | 1 mg/mL | 1 mL in a 2 mL multiple dose vial, in packages of 25. |
PROTECT FROM LIGHT.
Use only if solution is clear and seal intact.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
PACKAGE LABEL - PRINCIPAL DISPLAY - Scopolamine 1 mL Multiple Dose Vial Label
NDC 63323-268-01
26801
Scopolamine Hydrobromide Injection, USP
0.4 mg/mL
For IM, IV or SC Use
1 mL Multiple Dose Vial
Usual Dosage: See insert.
Protect From Light.

PACKAGE LABEL - PRINCIPAL DISPLAY - Scopolamine 1 mL Multiple Dose Vial Tray Label
NDC 63323-268-01
26801
Scopolamine Hydrobromide Injection, USP
0.4 mg/mL
For IM, IV or SC Use
1 mL Multiple Dose Vial
Rx only

| SCOPOLAMINE HYDROBROMIDE
scopolamine hydrobromide injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Fresenius Kabi USA, LLC (608775388) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fresenius Kabi USA, LLC | 840771732 | MANUFACTURE(63323-268) | |
