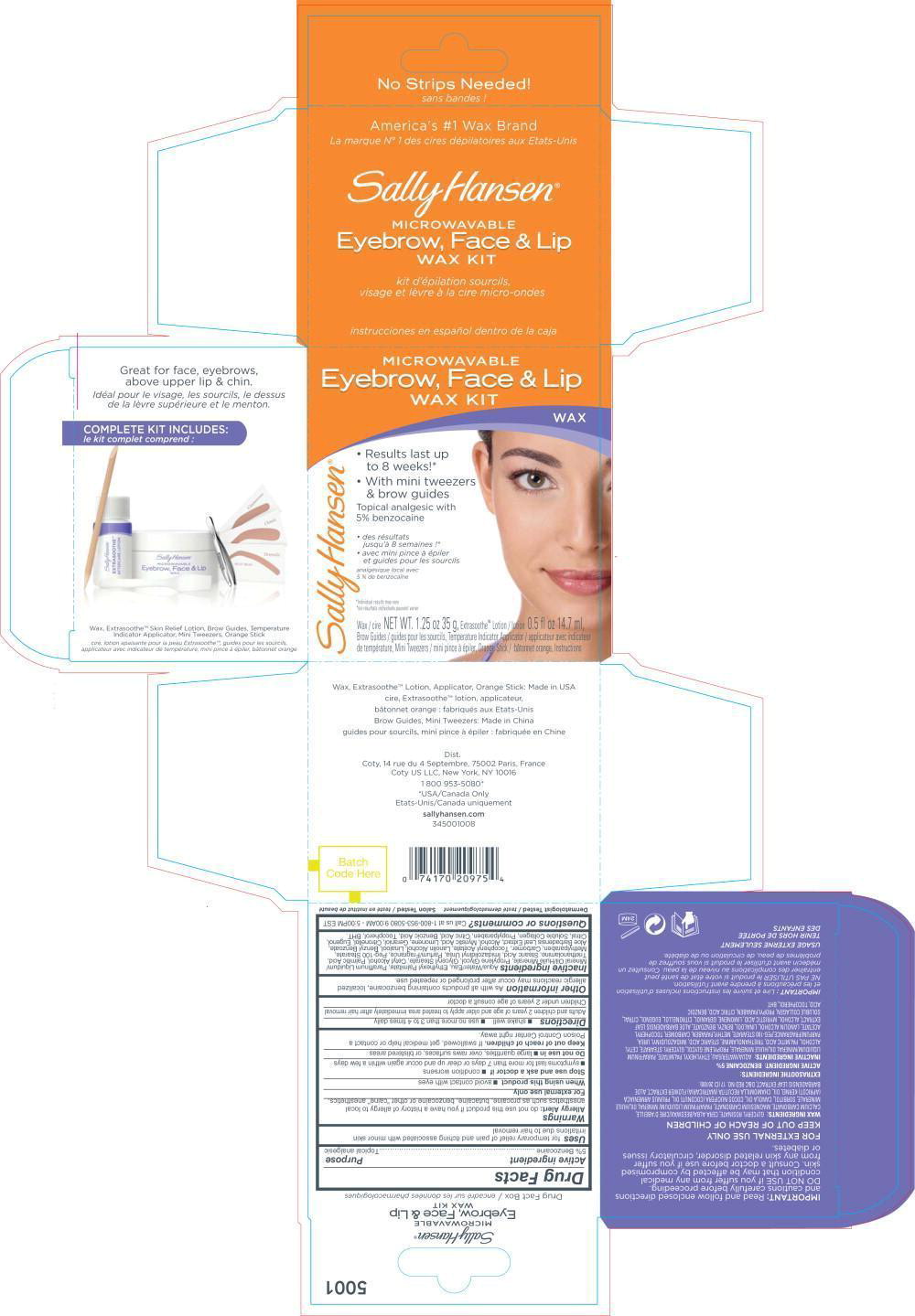
Label: SALLY HANSEN MICROWAVABLE EYBROW, FACE AND LIP WAX KIT- benzocaine kit
- NDC Code(s): 66184-155-01
- Packager: Coty US LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 25, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Allergy Alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
For external use only
- Directions
-
Inactive ingredients
Aqua/Water/Eau, Ethylhexyl Palmitate, Paraffinum Liquidum/ Mineral Oil/Huile Minerale, Propylene Glycol, Glyceryl Stearate, Cetyl Alcohol, Palmitic Acid, Triethanolamine, Stearic Acid, Imidazolidinyl Urea, Parfum/Fragrance, Peg-100 Stearate, Methylparaben, Carbomer, Tocopheryl Acetate, Lanolin Alcohol, Linalool, Benzyl Benzoate, Aloe Barbadensis Leaf Extract, Alcohol, Myristic Acid, Limonene, Geraniol, Citronellol, Eugenol, Citral, Soluble Collagen, Propylparaben, Citric Acid, Benzoic Acid, Tocopherol, BHT.
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Kit Label
MICROWABLE
Eyebrow, Face & Lip
WAX KITWAX
• Results last up
to 8 weeks!*
• With mini tweezers
& brow guidesTopical analgesic with
5 % benzocaine• des resultants
jusqu'à 8 semaines !*
• avec mini pince à épiler
et guides pour les sourcilsanalgésique local avec
5 % de benzocaïne*Individual results may vary
*les resultants individuals prevent varierWax / cire NET WT. 1.25 oz 35 g, Extrasoothe™ Lotion / lotion 0.5 fl oz 14.7 ml,
Brow Guides / guides pour les sourcils, Temperature Indicator Applicator . applicateur avec indicateur
de temperature, Mini Tweezers / mini pince à épiler, Orange Stick / bâtonnet orange, Instuctions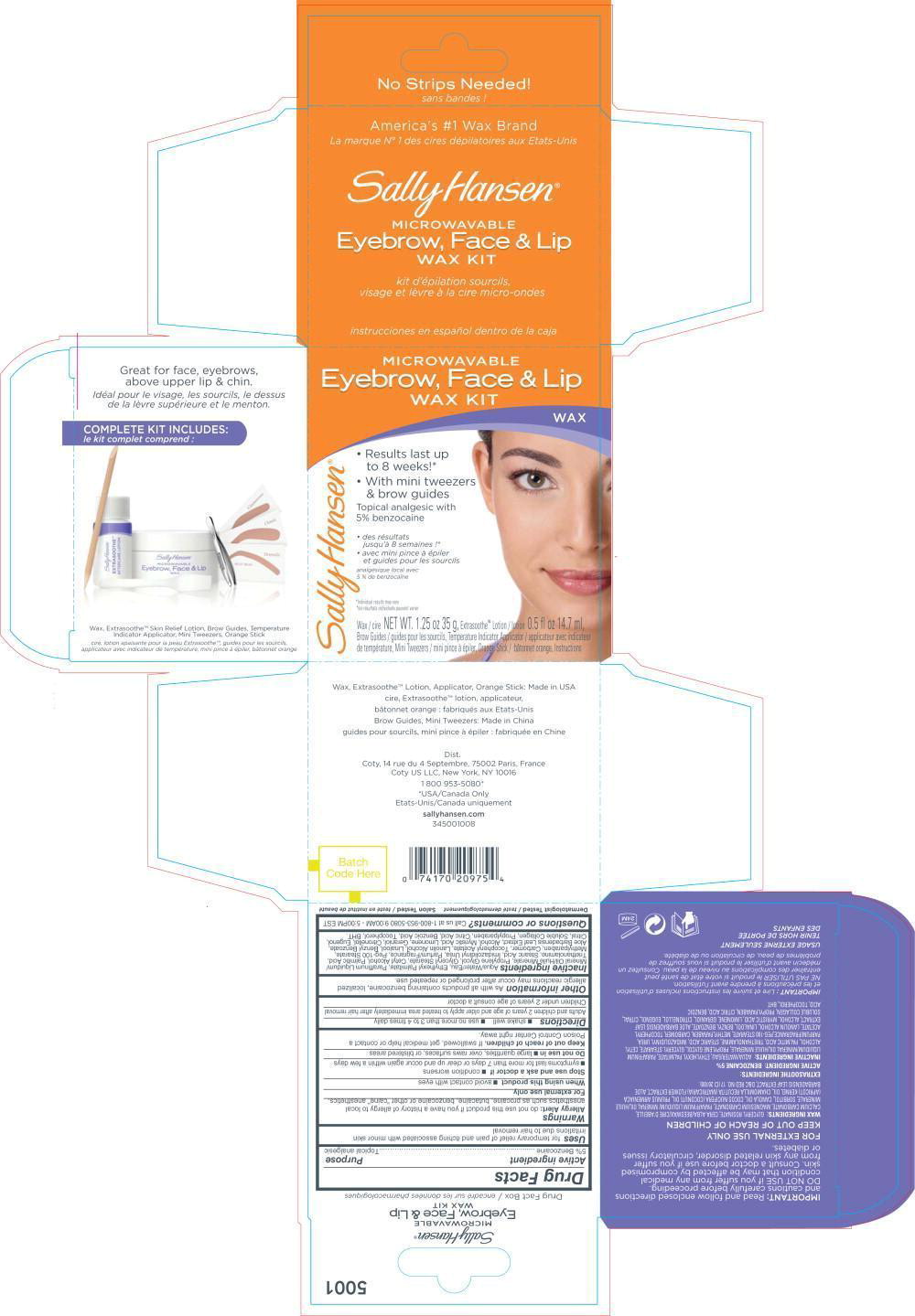
-
INGREDIENTS AND APPEARANCE
SALLY HANSEN MICROWAVABLE EYBROW, FACE AND LIP WAX KIT
benzocaine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66184-155 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66184-155-01 1 in 1 CARTON 01/01/2012 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 0.05 g Part 2 1 JAR 189.9 g Part 1 of 2 SALLY HANSEN MICROWAVABLE EYBROW, FACE AND LIP WAX KIT
benzocaine lotionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 0.05 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ETHYLHEXYL PALMITATE (UNII: 2865993309) MINERAL OIL (UNII: T5L8T28FGP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) PALMITIC ACID (UNII: 2V16EO95H1) TROLAMINE (UNII: 9O3K93S3TK) STEARIC ACID (UNII: 4ELV7Z65AP) IMIDUREA (UNII: M629807ATL) PEG-100 STEARATE (UNII: YD01N1999R) METHYLPARABEN (UNII: A2I8C7HI9T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) LINALOOL, (+/-)- (UNII: D81QY6I88E) BENZYL BENZOATE (UNII: N863NB338G) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALCOHOL (UNII: 3K9958V90M) MYRISTIC ACID (UNII: 0I3V7S25AW) GERANIOL (UNII: L837108USY) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) EUGENOL (UNII: 3T8H1794QW) CITRAL (UNII: T7EU0O9VPP) PROPYLPARABEN (UNII: Z8IX2SC1OH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BENZOIC ACID (UNII: 8SKN0B0MIM) TOCOPHEROL (UNII: R0ZB2556P8) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.05 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 01/01/2012 Part 2 of 2 SALLY HANSEN MICROWAVABLE EYBROW, FACE AND LIP WAX KIT
depilatories stripProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WHITE WAX (UNII: 7G1J5DA97F) INGR CALCIUM CARBONATE (UNII: H0G9379FGK) INGR MAGNESIUM CARBONATE (UNII: 0E53J927NA) INGR MINERAL OIL (UNII: T5L8T28FGP) INGR CANOLA OIL (UNII: 331KBJ17RK) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR APRICOT KERNEL OIL (UNII: 54JB35T06A) INGR CHAMOMILE (UNII: FGL3685T2X) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR D&C RED NO. 17 (UNII: ND733RX3JN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 189.9 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 01/01/2012 Labeler - Coty US LLC (039056361) Establishment Name Address ID/FEI Business Operations Crystal Claire Cosmetics 205493484 manufacture(66184-155)