TRIAL ANTACID- calcium carbonate tablet
Zee Medical Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
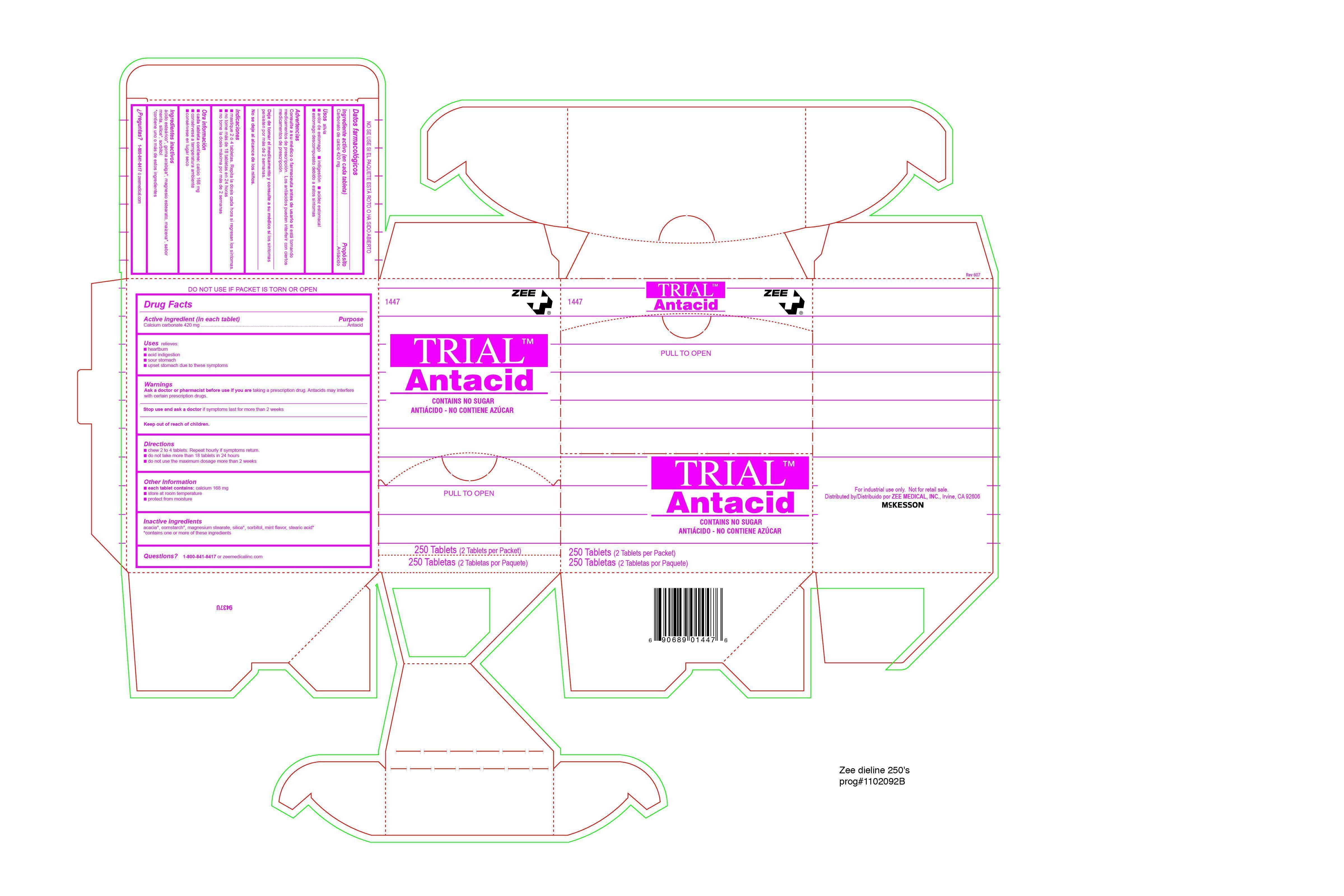
Drug Facts
Directions
- chew 2 to 4 tablets. Repeat hourly if symptoms return.
- do not take more than 18 tablets in 24 hours
- do not use the maximum dosage more than 2 weeks
Warnings
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interfere
with certain prescription drugs
KEEP OUT OF REACH OF CHILDREN. In case of overdose,
get medical help or contact a Poison Control Center right away.
Prompt medical attention is critical for adults as well as for
children even if you do not notice any signs or symptoms.
| TRIAL ANTACID
calcium carbonate tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Zee Medical Inc (009645623) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ultratab Laboratories, Inc. | 151051757 | manufacture(35418-310) | |
Revised: 1/2023
Document Id: f19c5d7f-48e7-d2c6-e053-2995a90a4255
Set id: a17b9b76-b932-4147-99b1-d3b1f42f175d
Version: 4
Effective Time: 20230106
Zee Medical Inc
 1
1