Label: SUPER SPOT REMOVER ACNE TREATMENT- salicylic acid gel
- NDC Code(s): 59427-005-01
- Packager: ORIGINS NATURAL RESOURCES INC.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 5, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
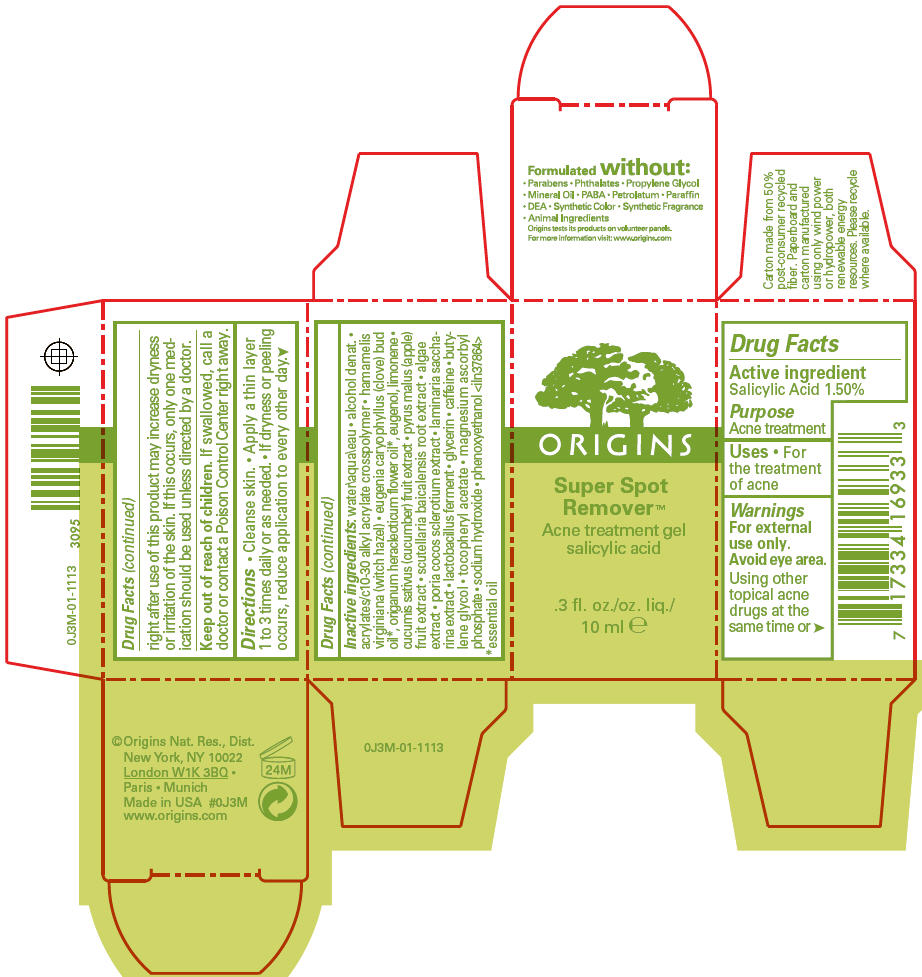
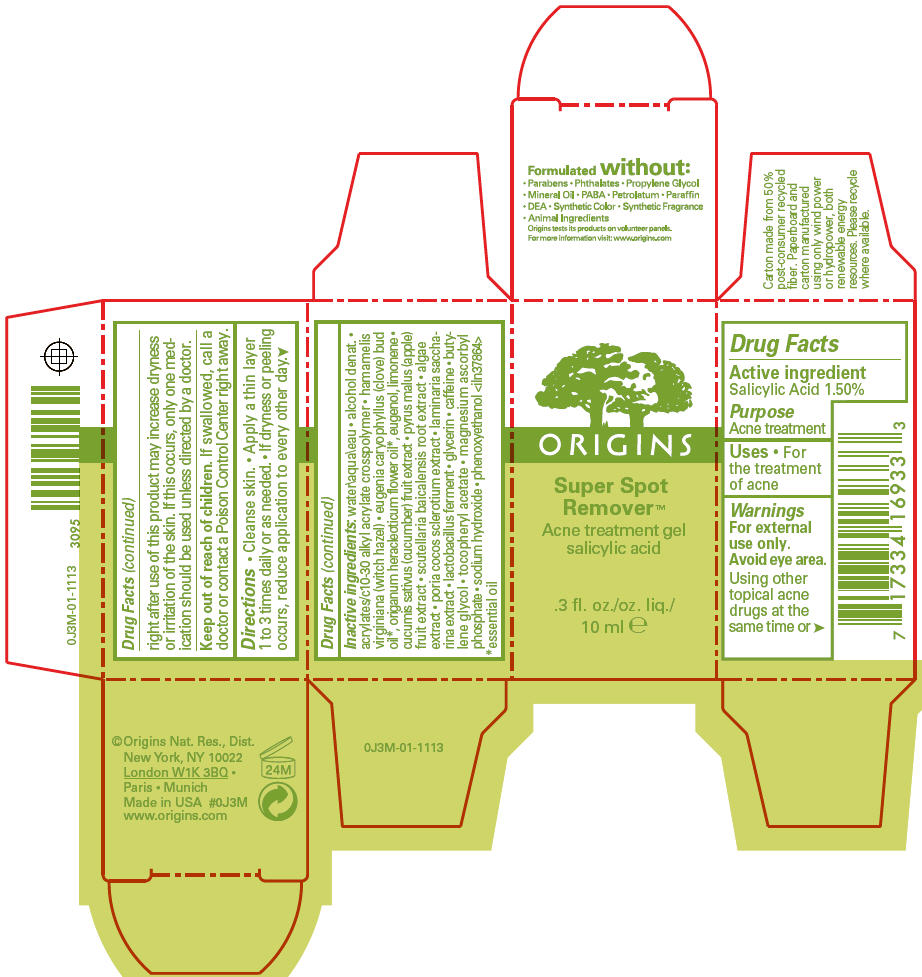
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water\aqua\eau • alcohol denat. • acrylates/c10-30 alkyl acrylate crosspolymer • hamamelis virginiana (witch hazel) • eugenia caryophyllus (clove) bud oil 1, origanum heracleoticum flower oil 1, eugenol, limonene • cucumis sativus (cucumber) fruit extract • pyrus malus (apple) fruit extract • scutellaria baicalensis root extract • algae extract • poria cocos sclerotium extract • laminaria saccharina extract • lactobacillus ferment • glycerin • caffeine • butylene glycol • tocopheryl acetate • magnesium ascorbyl phosphate • sodium hydroxide • phenoxyethanol <iln37864>
- 1
- essential oil
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 ml Carton
-
INGREDIENTS AND APPEARANCE
SUPER SPOT REMOVER ACNE TREATMENT
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59427-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength ORIGANUM VULGARE SUBSP. HIRTUM FLOWER (UNII: 3799SP11NY) WATER (UNII: 059QF0KO0R) WITCH HAZEL (UNII: 101I4J0U34) CLOVE OIL (UNII: 578389D6D0) EUGENOL (UNII: 3T8H1794QW) CUCUMBER (UNII: YY7C30VXJT) APPLE (UNII: B423VGH5S9) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) GLYCERIN (UNII: PDC6A3C0OX) CAFFEINE (UNII: 3G6A5W338E) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALCOHOL (UNII: 3K9958V90M) CARBOMER 1342 (UNII: 809Y72KV36) FU LING (UNII: XH37TWY5O4) LIMOSILACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) PORPHYRIDIUM PURPUREUM (UNII: K2P8K2558N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59427-005-01 1 in 1 CARTON 11/24/2020 1 10 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 04/01/2012 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Bentley Laboratories, LLC 068351753 manufacture(59427-005) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(59427-005)