Label: ACETAMINOPHEN tablet, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 37205-662-57, 37205-662-58 - Packager: Cardinal Health
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 16, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
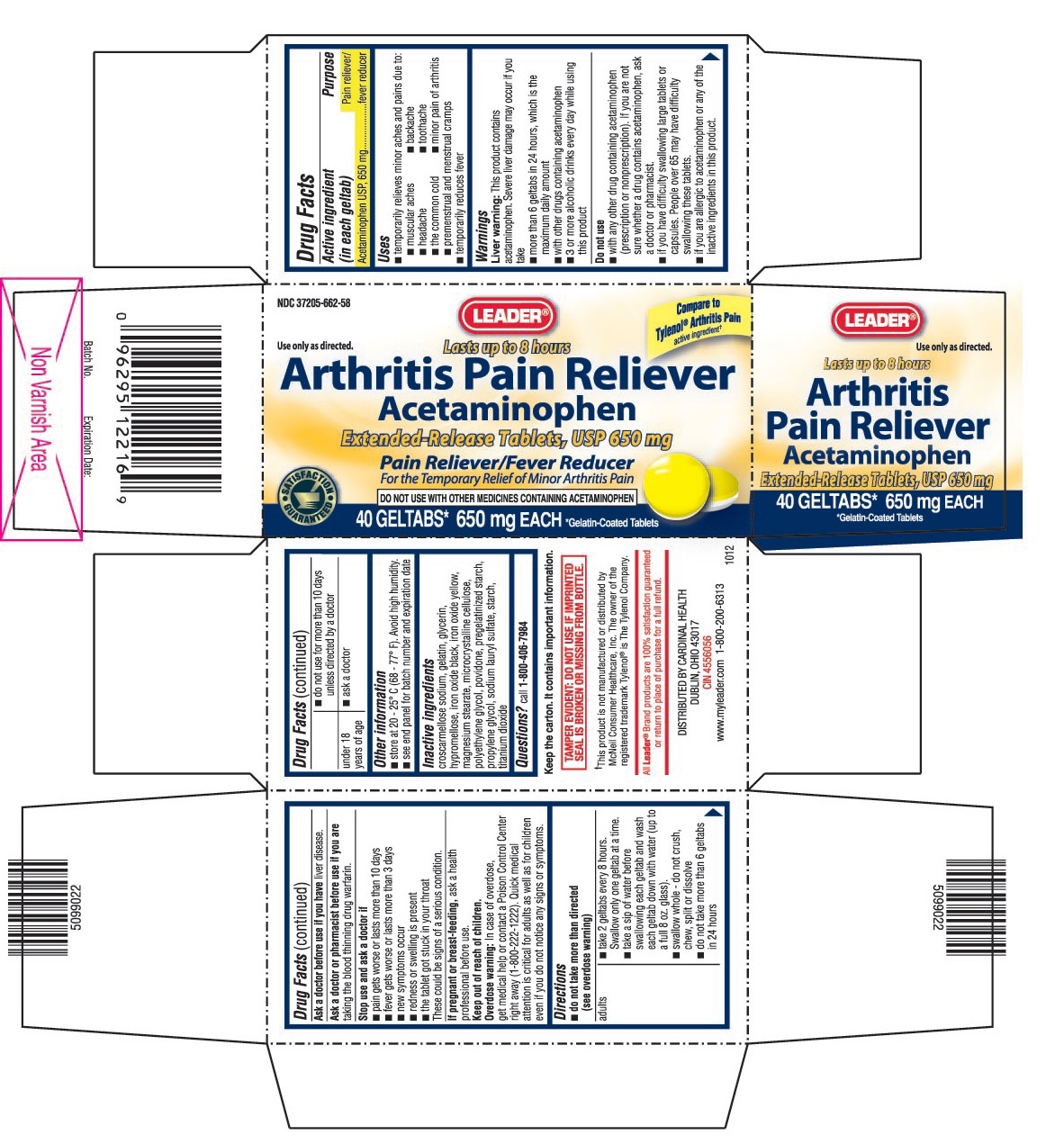
- ACTIVE INGREDIENT (IN EACH GELTAB)
- PURPOSE
- USES
-
WARNINGS
Liver warning: This product contains acetaminophen. Sever liver damage may occur if you take
- more than 6 geltabs in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have difficulty swallowing large tablets or capsules. People over 65 may have difficulty swallowing these tablets.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
-
DIRECTIONS
-
do not take more than directed (see overdose warning)
adults ▪ take 2 geltabs every 8 hours. Swallow only one geltab at a time.
▪ take a sip of water before swallowing each geltab and wash each geltab down with water (up to a full 8 oz. glass).
▪ swallow whole - do not crush, chew, split or dissolve
▪ do not take more than 6 geltabs in 24 hours
▪ do not use for more than 10 days unless directed by a doctorunder 18 years of age ▪ ask a doctor
-
do not take more than directed (see overdose warning)
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37205-662 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSES (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (White to Yellow) Score no score Shape ROUND Size 13mm Flavor Imprint Code 350 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37205-662-58 40 in 1 BOTTLE 2 NDC:37205-662-57 80 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078569 12/21/2012 Labeler - Cardinal Health (097537435) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 184769029 manufacture(37205-662)