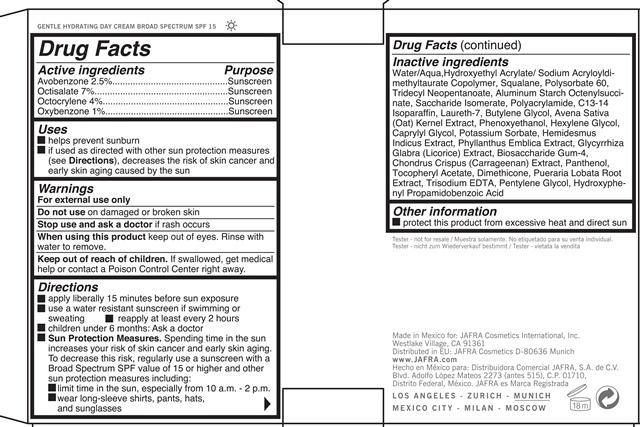
When using this project keep out of eyes. Rinse with water to remove.
Directions
apply liberally 15 minutes before sun exposure
use a water resistant sunscreen if swimming or sweating
reapply at least every 2 hours
children under 6 months: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk. regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures , including:
limit time in the sun, especially from 10 a.m. - 2 p.m.
wear long-sleeve shirts, pants, hats and sunglasses
Inactive ingredients: Water/aqua, C12-15 Alkyl Benzoate, Hydroxyethyl Acrylate/ Sodium Acryloyl dimethyltaurate Copolymer, Squalane, Polysorbate 60, Tridecyl Neopentanoate, Aluminum starch Octenylsuccinate, Saccharide Isomerate, Polyacrylamide, C13-14 Isoparrafin, Laureth-7, Butylene Glycol, Avena Sativa (Oat) Kernel Extract, Phenoxyethanol, Hexylene Glycol, Caprylyl Glycol, Potassium Sorbate, Hemidesmus Indicus Extract, Phyllanthus Emblica Extract, Glycrrhiza Glabra (licorice) Extract, Biosaccharide Gum-4, Chondrus Crispus (Carrageenan) Extract, Panthenol, Tocopheryl Acetate, Dimethicone, Pueraria Lobata Root Extract, Trisodium EDTA, Pentylene Glycol, Propamidobenzoic acid.