Label: ACETALDEHYDE- acetaldehyde spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 63776-605-14 - Packager: VIATREXX BIO INCORPORATED
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 24, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose:
- Uses
- Warnings
- Dosage
- Other Ingredients
- Other Information
- Questions
-
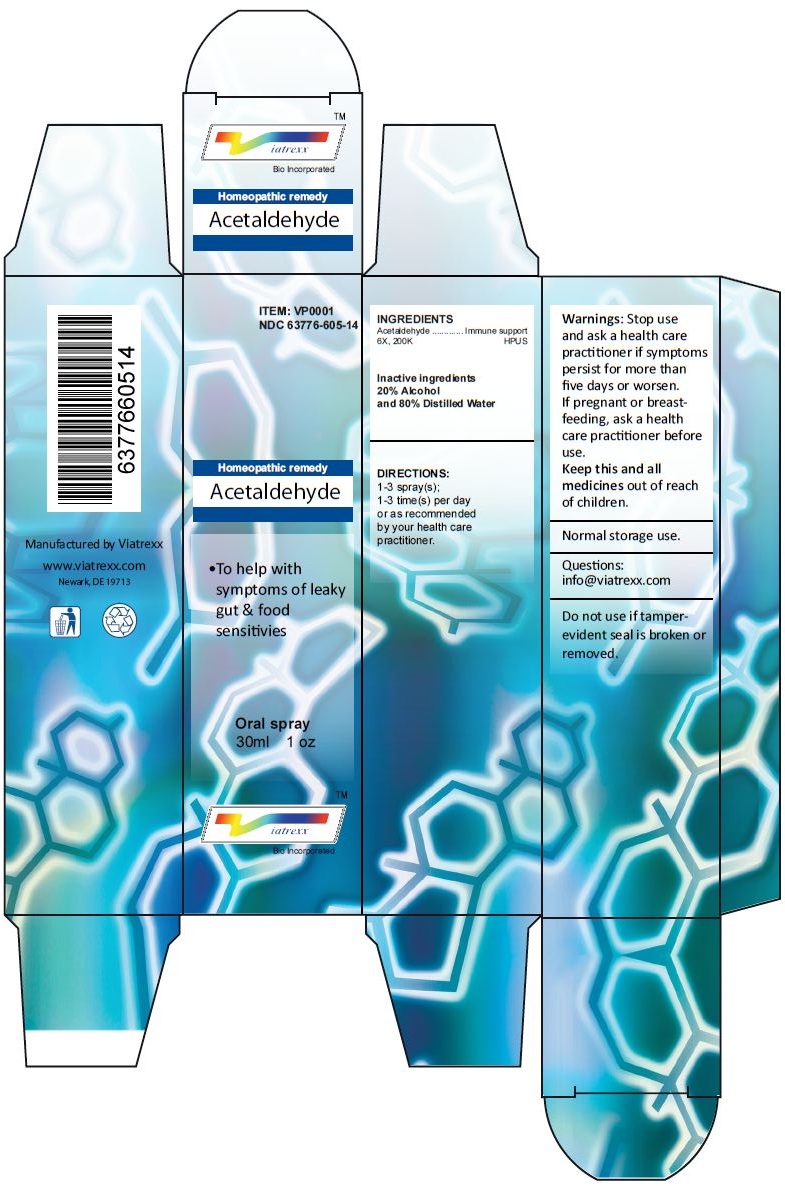
Principal Display Panel
ITEM: VP0001
NDC 63776-605-14
Homeopathic remedy
Acetaldehyde
• To help with symptoms of leaky gut & food sensitivies
Oral spray
30ml 1 oz
Viatrexx™ Bio Incorporated
Manufactured by Viatrexx
www.viatrexx.com
Newark, DE 19713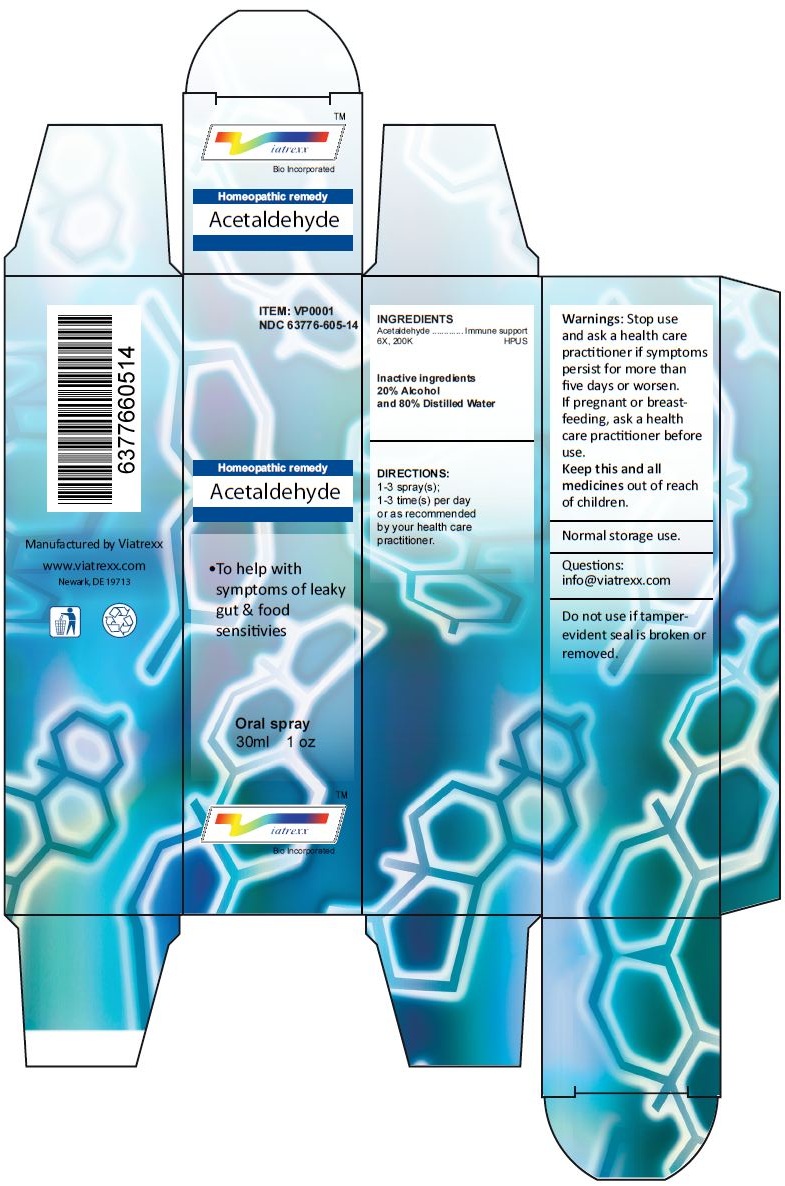
Acetaldehyde
30 ml
1 oz
Viatrexx™ Bio Incorporated
ITEM: VP0001
NDC: 63776-605-14
INDICATIONS:
To help with symptoms of leaky gut & food sensitivies
DIRECTIONS:
1-3 spray(s); 1-3 time(s) per day or as recommended by your health care practitioner.
Mfg. for
Viatrexx Bio Incorporated.
www.viatrexx.com
Newark, DE 19713
-
INGREDIENTS AND APPEARANCE
ACETALDEHYDE
acetaldehyde sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63776-605 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Acetaldehyde (UNII: GO1N1ZPR3B) (Acetaldehyde - UNII:GO1N1ZPR3B) Acetaldehyde 200 {kp_C} in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63776-605-14 1 in 1 BOX 1 30 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/24/2012 Labeler - VIATREXX BIO INCORPORATED (078419880) Establishment Name Address ID/FEI Business Operations Les Importations Herbasante Inc 243254612 MANUFACTURE(63776-605)