RAVICTI- glycerol phenylbutyrate liquid
Hyperion Therapeutics, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use
RAVICTI safely and effectively. See full prescribing information for RAVICTI. RAVICTI™ (glycerol phenylbutyrate) oral liquid Initial U.S. Approval: 1996 INDICATIONS AND USAGERAVICTI is indicated for use as a nitrogen-binding agent for chronic management of adult and pediatric patients ≥2 years of age with urea cycle disorders (UCDs) that cannot be managed by dietary protein restriction and/or amino acid supplementation alone. RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (e.g., essential amino acids, arginine, citrulline, protein-free calorie supplements). (1) Limitations of Use: DOSAGE AND ADMINISTRATIONRAVICTI should be prescribed by a physician experienced in management of UCDs. (2.1)
Switching From Sodium Phenylbutyrate to RAVICTI:
Initial Dosage in Phenylbutyrate-Naïve Patients:
Dosage Modifications in Patients With Hepatic Impairment: DOSAGE FORMS AND STRENGTHSOral liquid: 1.1 g/mL of glycerol phenylbutyrate. (3) WARNINGS AND PRECAUTIONSADVERSE REACTIONSMost common adverse reactions in ≥10% of patients are diarrhea, flatulence, and headache. (6) To report SUSPECTED ADVERSE REACTIONS, contact Hyperion Therapeutics at 1-855-823-7878 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 2/2013 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
RAVICTI is indicated for use as a nitrogen-binding agent for chronic management of adult and pediatric patients ≥2 years of age with urea cycle disorders (UCDs) who cannot be managed by dietary protein restriction and/or amino acid supplementation alone. RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (e.g., essential amino acids, arginine, citrulline, protein-free calorie supplements).
Limitations of Use:
RAVICTI is not indicated for the treatment of acute hyperammonemia in patients with UCDs because more rapidly acting interventions are essential to reduce plasma ammonia levels.
The safety and efficacy of RAVICTI for the treatment of N-acetylglutamate synthase (NAGS) deficiency has not been established.
The use of RAVICTI in patients <2 months of age is contraindicated [see Contraindications (4)].
2 DOSAGE AND ADMINISTRATION
2.1 Important Instructions
RAVICTI should be prescribed by a physician experienced in the management of UCDs. Instruct patients to take RAVICTI with food and to administer directly into the mouth via oral syringe or dosing cup. See the instructions on the use of RAVICTI by nasogastric tube or g-tube [see Dosage and Administration (2.6)].
The recommended dosages for patients switching from sodium phenylbutyrate to RAVICTI and patients naïve to phenylbutyric acid are different [see Dosage and Administration (2.2, 2.3)]. For both subpopulations:
- Give RAVICTI in 3 equally divided dosages, each rounded up to the nearest 0.5 mL.
- The maximum total daily dosage is 17.5 mL (19 g).
- RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (e.g., essential amino acids, arginine, citrulline, protein-free calorie supplements).
2.2 Switching From Sodium Phenylbutyrate to RAVICTI
Patients switching from sodium phenylbutyrate to RAVICTI should receive the dosage of RAVICTI that contains the same amount of phenylbutyric acid. The conversion is as follows:.
Total daily dosage of RAVICTI (mL) = total daily dosage of sodium phenylbutyrate (g) x 0.86
2.3 Initial Dosage in Phenylbutyrate-Naïve Patients
The recommended dosage range, based upon body surface area, in patients naïve to phenylbutyrate (PBA) is 4.5 to 11.2 mL/m2/day (5 to 12.4 g/m2/day). For patients with some residual enzyme activity who are not adequately controlled with protein restriction, the recommended starting dosage is 4.5 mL/m2/day.
In determining the starting dosage of RAVICTI in treatment-naïve patients, consider the patient's residual urea synthetic capacity, dietary protein requirements, and diet adherence. Dietary protein is approximately 16% nitrogen by weight. Given that approximately 47% of dietary nitrogen is excreted as waste and approximately 70% of an administered PBA dose will be converted to urinary phenylacetylglutamine (U-PAGN), an initial estimated RAVICTI dose for a 24-hour period is 0.6 mL RAVICTI per gram of dietary protein ingested per 24 hour period. The total daily dosage should not exceed 17.5 mL.
2.4 Dosage Adjustment and Monitoring
Adjustment based on Plasma Ammonia: Adjust the RAVICTI dosage to produce a fasting plasma ammonia level that is less than half the upper limit of normal (ULN) according to age.
Adjustment Based on Urinary Phenylacetylglutamine: If available, U-PAGN measurements may be used to help guide RAVICTI dose adjustment. Each gram of U-PAGN excreted over 24 hours covers waste nitrogen generated from 1.4 grams of dietary protein. If U-PAGN excretion is insufficient to cover daily dietary protein intake and the fasting ammonia is greater than half the ULN, the RAVICTI dose should be adjusted upward. The amount of dose adjustment should factor in the amount of dietary protein that has not been covered, as indicated by the 24-h U-PAGN level and the estimated RAVICTI dose needed per gram of dietary protein ingested and the maximum total daily dosage i.e., 17.5 mL.
Consider a patient's use of concomitant medications, such as probenecid, when making dosage adjustment decisions based on U-PAGN. Probenecid may result in a decrease of the urinary excretion of PAGN [see Drug Interactions (7.2)].
Adjustment Based on Plasma Phenylacetate: If available, measurements of the plasma PAA levels may be useful to guide dosing if symptoms of vomiting, nausea, headache, somnolence, confusion, or sleepiness are present in the absence of high ammonia or intercurrent illness. Ammonia levels must be monitored closely when changing the dose of RAVICTI. The ratio of PAA to PAGN in plasma may provide additional information to assist in dose adjustment decisions. In patients with a high PAA to PAGN ratio, a further increase in RAVICTI dose may not increase PAGN formation, even if plasma PAA concentrations are increased, due to saturation of the conjugation reaction. The PAA to PAGN ratio has been observed to be generally less than 1 in patients without significant PAA accumulation [see Warnings and Precautions (5.1)].
2.5 Dosage Modifications in Patients with Hepatic Impairment
For patients with moderate to severe hepatic impairment, the recommended starting dosage is at the lower end of the range [see Warnings and Precautions (5.1) and Use in Specific Populations (8.6)].
2.6 Preparation for Nasogastric Tube or Gastrostomy Tube Administration
For patients who have a nasogastric tube or gastrostomy tube in place, administer RAVICTI as follows:
- Utilize an oral syringe to withdraw the prescribed dosage of RAVICTI from the bottle.
- Place the tip of the syringe into to the tip of the gastrostomy/nasogastric tube.
- Utilizing the plunger of the syringe, administer RAVICTI into the tube.
- Flush once with 30 mL of water and allow the flush to drain.
- Flush a second time with an additional 30 mL of water to clear the tube.
3 DOSAGE FORMS AND STRENGTHS
Oral liquid: colorless to pale yellow, 1.1 g/mL of glycerol phenylbutyrate (delivers 1.02 g/mL of phenylbutyrate).
4. CONTRAINDICATIONS
RAVICTI is contraindicated in patients
- Less than 2 months of age. Children <2 months of age may have immature pancreatic exocrine function, which could impair hydrolysis of RAVICTI, leading to impaired absorption of phenylbutyrate and hyperammonemia [see Pediatric Use (8.4)].
- With known hypersensitivity to phenylbutyrate. Signs of hypersensitivity include wheezing, dyspnea, coughing, hypotension, flushing, nausea, and rash.
5 WARNINGS AND PRECAUTIONS
5.1 Neurotoxicity
The major metabolite of RAVICTI, PAA, is associated with neurotoxicity. Signs and symptoms of PAA neurotoxicity, including somnolence, fatigue, lightheadedness, headache, dysgeusia, hypoacusis, disorientation, impaired memory, and exacerbation of preexisting neuropathy, were observed at plasma PAA concentrations ≥500 µg/mL in a study of cancer patients who were administered IV PAA. In this study, adverse events were reversible.
In healthy subjects, after administration of 4 mL and 6 mL RAVICTI 3 times daily for 3 days, a dose-dependent increase in all-grade nervous system adverse reactions was observed, even at exposure levels of PAA <100 µg/mL.
In clinical trials in UCD patients who had been on sodium phenylbutyrate prior to administration of RAVICTI, peak PAA concentrations after dosing with RAVICTI ranged from 1.6 to 178 µg/mL (mean: 39 µg/mL) in adult patients and from 7 to 480 µg/mL (mean: 90 µg/mL) in pediatric patients. Some UCD patients experienced headache, fatigue, symptoms of peripheral neuropathy, seizures, tremor and/or dizziness. No correlation between PAA levels and neurotoxicity symptoms was identified but PAA levels were generally not measured at the time of neurotoxicity symptoms.
If symptoms of vomiting, nausea, headache, somnolence, confusion, or sleepiness are present in the absence of high ammonia or other intercurrent illnesses, reduce the RAVICTI dosage.
5.2 Reduced Phenylbutyrate Absorption in Pancreatic Insufficiency or Intestinal Malabsorption
Exocrine pancreatic enzymes hydrolyze RAVICTI in the small intestine, separating the active moiety, phenylbutyrate, from glycerol. This process allows phenylbutyrate to be absorbed into the circulation. Low or absent pancreatic enzymes or intestinal disease resulting in fat malabsorption may result in reduced or absent digestion of RAVICTI and/or absorption of phenylbutyrate and reduced control of plasma ammonia. Monitor ammonia levels closely in patients with pancreatic insufficiency or intestinal malabsorption.
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Assessment of adverse reactions was based on exposure of 45 adult patients (31 female and 14 male) with UCD subtype deficiencies of ornithine transcarbamylase (OTC, n=40), carbamyl phosphate synthetase (CPS, n=2), and argininosuccinate synthetase (ASS, n=1) in a randomized, double-blind, active-controlled (RAVICTI vs sodium phenylbutyrate), crossover, 4-week study (Study 1) that enrolled patients ≥18 years of age [see Clinical Studies (14.1)]. One of the 45 patients received only sodium phenylbutyrate prior to withdrawing on day 1 of the study due to an adverse reaction.
Table 1 summarizes adverse reactions occurring in ≥2 patients treated with RAVICTI or sodium phenylbutyrate. The most common adverse reactions (occurring in ≥10% of patients) reported during short-term treatment with RAVICTI were diarrhea, flatulence, and headache.
| Number (%) of Patients in Study 1 | ||||||||||
| Sodium Phenylbutyrate (N = 45) | RAVICTI (N = 44) |
|||||||||
| Gastrointestinal disorders | ||||||||||
| Abdominal discomfort | 3 (7) | 0 | ||||||||
| Abdominal pain | 2 (4) | 3 (7) | ||||||||
| Diarrhea | 3 (7) | 7 (16) | ||||||||
| Dyspepsia | 3 (7) | 2 (5) | ||||||||
| Flatulence | 1 (2) | 6 (14) | ||||||||
| Nausea | 3 (7) | 1 (2) | ||||||||
| Vomiting | 2 (4) | 3 (7) | ||||||||
| General disorders and administration site conditions | ||||||||||
| Fatigue | 1 (2) | 3 (7) | ||||||||
| Investigations | ||||||||||
| Ammonia increased | 1 (2) | 2 (5) | ||||||||
| Metabolism and nutrition disorders | ||||||||||
| Decreased appetite | 2 (4) | 3 (7) | ||||||||
| Nervous system disorders | ||||||||||
| Dizziness | 4 (9) | 0 | ||||||||
| Headache | 4 (9) | 6 (14) | ||||||||
Other Adverse Reactions
RAVICTI has been evaluated in 77 UCD patients (51 adult and 26 pediatric) in 2 open-label long-term studies, in which 69 patients completed 12 months of treatment with RAVICTI (median exposure = 51 weeks). During these studies there were no deaths.
Adverse reactions occurring in ≥10% of adult patients were nausea, vomiting, diarrhea, decreased appetite, hyperammonemia, dizziness, headache, and fatigue.
Adverse reactions occurring in ≥10% of pediatric patients were upper abdominal pain, rash, nausea, vomiting, diarrhea, decreased appetite, hyperammonemia, and headache.
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect Ammonia
Corticosteroids
Use of corticosteroids may cause the breakdown of body protein and increase plasma ammonia levels. Monitor ammonia levels closely when corticosteroids and RAVICTI are used concomitantly.
Valproic Acid and Haloperidol
Hyperammonemia may be induced by haloperidol and by valproic acid. Monitor ammonia levels closely when use of valproic acid or haloperidol is necessary in UCD patients.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
A voluntary patient registry will include evaluation of pregnancy outcomes in patients with UCDs. For more information regarding the registry program, visit www.ucdregistry.com or call 1-855-823-2595.
Pregnancy Category C
Risk Summary
There are no adequate and well-controlled studies in pregnant women. In rabbits given glycerol phenylbutyrate at doses up to 2.7 times the dose of 6.87 mL/m2/day in adult patients (based on combined area under the curve [AUCs] for PBA and PAA) during the period of organogenesis, maternal toxicity, but no effects on embryo-fetal development, was observed. In rats given glycerol phenylbutyrate at 1.9 times the dose of 6.87 mL/m2/day in adult patients (based on combined AUCs for PBA and PAA), no adverse embryo-fetal effects were observed. Maternal toxicity, reduced fetal weights, and variations in skeletal development were observed in rats at doses greater than or equal to 5.7 times the dose of 6.87 mL/m2/day in adult patients (based on combined AUCs for PBA and PAA). RAVICTI should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
Oral administration of glycerol phenylbutyrate during the period of organogenesis up to 350 mg/kg/day in rabbits produced maternal toxicity, but no effects on embryo-fetal development. The dose of 350 mg/kg/day in rabbits is approximately 2.7 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA. In rats, at an oral dose of 300 mg/kg/day of glycerol phenylbutyrate (1.9 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) during the period of organogenesis, no effects on embryo-fetal development were observed. Doses ≥650 mg/kg/day produced maternal toxicity and adverse effects on embryo-fetal development including reduced fetal weights and cervical ribs at the 7th cervical vertebra. The dose of 650 mg/kg/day in rats is approximately 5.7 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA. No developmental abnormalities, effects on growth, or effects on learning and memory were observed in rats through day 92 postpartum following oral administration in pregnant rats with up to 900 mg/kg/day of glycerol phenylbutyrate (8.5 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) during organogenesis and lactation.
8.3 Nursing mothers
It is not known whether RAVICTI or its metabolites are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions from RAVICTI in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into consideration the importance of the drug to the health of the mother.
8.4 Pediatric use
Patients Between 2 and <18 Years of Age
The safety and efficacy of RAVICTI in patients 2 to <18 years of age were established in 2 open-label, sodium phenylbutyrate to RAVICTI, fixed-sequence, switchover clinical trials [see Adverse Reactions (6) and Clinical Studies (14.2)].
Patients ≥2 Months and <2 Years of Age
The safety and efficacy of RAVICTI in patients 2 months to <2 years of age has not been established. PK and ammonia control were studied in only 4 patients between 2 months and <2 years of age, providing insufficient data to establish a safe and effective dose in this age range.
Patients <2 Months of Age
RAVICTI is contraindicated in patients <2 months of age [see Contraindications (4)]. Children <2 months of age may have immature pancreatic exocrine function, which could impair hydrolysis of RAVICTI. Pancreatic lipases may be necessary for intestinal hydrolysis of RAVICTI, allowing release of phenylbutyrate and subsequent formation of PAA, the active moiety. It is not known whether pancreatic and extrapancreatic lipases are sufficient for hydrolysis of RAVICTI. If there is inadequate intestinal hydrolysis of RAVICTI, impaired absorption of phenylbutyrate and hyperammonemia could occur.
Juvenile Animal Study
In a juvenile rat study with daily oral dosing performed on postpartum day 2 through mating and pregnancy after maturation, terminal body weight was dose-dependently reduced by up to 16% in males and 12% in females. Learning, memory, and motor activity endpoints were not affected. However, fertility (number of pregnant rats) was decreased by up to 25% at ≥650 mg/kg/day (2.6 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA). Embryo toxicity (increased resorptions) occurred at 650 mg/kg/day (2.6 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) and litter size was reduced at 900 mg/kg/day (3 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA).
8.5 Geriatric use
Clinical studies of RAVICTI did not include sufficient numbers of subjects ≥65 years of age to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
No studies were conducted in UCD patients with hepatic impairment. Because conversion of PAA to PAGN occurs in the liver, patients with hepatic impairment may have reduced conversion capability and higher plasma PAA and PAA to PAGN ratio. Therefore, dosage for patients with moderate to severe hepatic impairment should be started at the lower end of the recommended dosing range and should be kept on the lowest dose necessary to control their ammonia levels [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
While there is no experience with overdosage in human clinical trials, PAA, a toxic metabolite of RAVICTI, can accumulate in patients who receive an overdose. In case of overdosage, discontinue the drug and contact poison control [see Warnings and Precautions (5.1)].
11. DESCRIPTION
RAVICTI (glycerol phenylbutyrate) is a clear, colorless to pale yellow oral liquid. It is insoluble in water and most organic solvents, and it is soluble in dimethylsulfoxide (DMSO) and >65% acetonitrile.
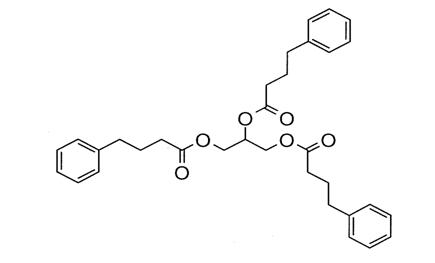
Glycerol phenylbutyrate is a nitrogen-binding agent. It is a triglyceride containing 3 molecules of PBA linked to a glycerol backbone, the chemical name of which is benzenebutanoic acid, 1', 1' ' –(1,2,3-propanetriyl) ester with a molecular weight of 530.67. It has a molecular formula of C33H38O6. The structural formula is:
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of action
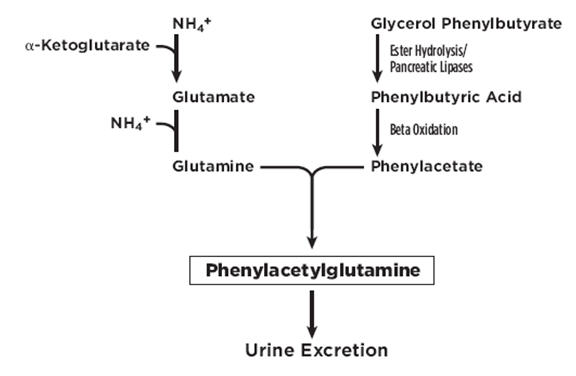
UCDs are inherited deficiencies of enzymes or transporters necessary for the synthesis of urea from ammonia (NH3, NH4+). Absence of these enzymes or transporters results in the accumulation of toxic levels of ammonia in the blood and brain of affected patients. RAVICTI is a triglyceride containing 3 molecules of phenylbutyrate (PBA). PAA, the major metabolite of PBA, is the active moiety of RAVICTI. PAA conjugates with glutamine (which contains 2 molecules of nitrogen) via acetylation in the liver and kidneys to form PAGN, which is excreted by the kidneys (Figure 1). On a molar basis, PAGN, like urea, contains 2 moles of nitrogen and provides an alternate vehicle for waste nitrogen excretion.
Figure 1: RAVICTI Mechanism of Action
12.2 Pharmacodynamics
Pharmacological Effects
In clinical studies, total 24-hour AUC of ammonia concentration was comparable at steady state during the switchover period between RAVICTI and sodium phenylbutyrate [see Clinical Studies (14)].
Cardiac Electrophysiology
The effect of multiple doses of RAVICTI 13.2 g/day and 19.8 g/day (approximately 69% and 104% of the maximum recommended daily dosage) on QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg), four-treatment-arm, crossover study in 57 healthy subjects. The upper bound of the one-sided 95% CI for the largest placebo-adjusted, baseline-corrected QTc, based on individual correction method (QTcI) for RAVICTI, was below 10 ms. However, assay sensitivity was not established in this study because the moxifloxacin time-profile was not consistent with expectation. Therefore, an increase in mean QTc interval of 10 ms cannot be ruled out.
12.3 Pharmacokinetics
Absorption
RAVICTI is a pro-drug of PBA. Upon oral ingestion, PBA is released from the glycerol backbone in the gastrointestinal tract by lipases. PBA derived from RAVICTI is further converted by β-oxidation to PAA.
In healthy, fasting adult subjects receiving a single oral dose of 2.9 mL/m2 of RAVICTI, peak plasma levels of PBA, PAA, and PAGN occurred at 2 h, 4 h, and 4 h, respectively. Upon single-dose administration of RAVICTI, plasma concentrations of PBA were quantifiable in 15 of 22 participants at the first sample time postdose (0.25 h). Mean maximum concentration (Cmax) for PBA, PAA, and PAGN was 37.0 µg/mL, 14.9 µg/mL, and 30.2 µg/mL, respectively. In healthy subjects, intact glycerol phenylbutyrate was detected in plasma. While the study was inconclusive, the incomplete hydrolysis of glycerol phenylbutyrate cannot be ruled out.
In healthy subjects, the systemic exposure to PAA, PBA, and PAGN increased in a dose-dependent manner. Following 4 mL of RAVICTI for 3 days (3 times a day [TID]), mean Cmax and AUC were 66 µg/mL and 930 µg•h/mL for PBA and 28 µg/mL and 942 µg•h/mL for PAA, respectively. In the same study, following 6 mL of RAVICTI for 3 days (TID), mean Cmax and AUC were 100µg/mL and 1400 µg•h/mL for PBA and 65 µg/mL and 2064 µg•h/mL for PAA, respectively.
In adult UCD patients receiving multiple doses of RAVICTI, maximum plasma concentrations at steady state (Cmaxss) of PBA, PAA, and PAGN occurred at 8 h, 12 h, and 10 h, respectively, after the first dose in the day. Intact glycerol phenylbutyrate was not detectable in plasma in UCD patients.
Distribution
In vitro, the extent of plasma protein binding for 14C-labeled metabolites was 80.6% to 98.0% for PBA (over 1-250 µg/mL), and 37.1% to 65.6% for PAA (over 5-500 µg/mL). The protein binding for PAGN was 7% to 12% and no concentration effects were noted.
Metabolism
Upon oral administration, pancreatic lipases hydrolyze RAVICTI (i.e., glycerol phenylbutyrate), and release PBA. PBA undergoes β-oxidation to PAA, which is conjugated with glutamine in the liver and in the kidney through the enzyme phenylacetyl-CoA: L-glutamine-N-acetyltransferase to form PAGN. PAGN is subsequently eliminated in the urine.
Saturation of conjugation of PAA and glutamine to form PAGN was suggested by increases in the ratio of plasma PAA to PAGN with increasing dose and with increasing severity of hepatic impairment.
In healthy subjects, after administration of 4 mL, 6 mL, and 9 mL 3 times daily for 3 days, the ratio of mean AUC0-23h of PAA to PAGN was 1, 1.25, and 1.6, respectively. In a separate study, in patients with hepatic impairment (Child-Pugh B and C), the ratios of mean Cmax values for PAA to PAGN among all patients dosed with 6 mL and 9 mL twice daily were 3 and 3.7.
In in vitro studies, the specific activity of lipases for glycerol phenylbutyrate was in the following decreasing order: pancreatic triglyceride lipase, carboxyl ester lipase, and pancreatic lipase–related protein 2. Further, glycerol phenylbutyrate was hydrolyzed in vitro by esterases in human plasma. In these in vitro studies, a complete disappearance of glycerol phenylbutyrate did not produce molar equivalent PBA, suggesting the formation of mono- or bis-ester metabolites. However, the formation of mono- or bis-esters was not studied in humans.
Excretion
The mean (SD) percentage of administered PBA excreted as PAGN was approximately 68.9% (17.2) in adults and 66.4% (23.9) in pediatric UCD patients at steady state. PAA and PBA represented minor urinary metabolites, each accounting for <1% of the administered dose of PBA.
Specific Population
Gender
In healthy adult volunteers, a gender effect was found for all metabolites, with women generally having higher plasma concentrations of all metabolites than men at a given dose level. In healthy female volunteers, mean Cmax for PAA was 51 and 120% higher than in male volunteers after administration of 4 mL and 6 mL 3 times daily for 3 days, respectively. The dose normalized mean AUC0-23h for PAA was 108% higher in females than in males.
Pediatrics
Population pharmacokinetic modeling and dosing simulations suggest body surface area to be the most significant covariate explaining the variability of PAA clearance. PAA clearance was 10.9 L/h, 16.4 L/h, and 24.4 L/h, respectively, for UCD patients ages 3 to 5, 6 to 11, and 12 to 17 years
Hepatic Impairment
The effects of hepatic impairment on the pharmacokinetics of RAVICTI were studied in patients with hepatic impairment of Child-Pugh A, B, and C receiving 100 mg/kg of RAVICTI twice daily for 7 days.
Plasma glycerol phenylbutyrate was not measured in patients with hepatic impairment.
After multiple doses of RAVICTI in patients with hepatic impairment of Child-Pugh A, B, and C, geometric mean AUCt of PBA was 42%, 84%, and 50% higher, respectively, while geometric mean AUCt of PAA was 22%, 53%, and 94% higher, respectively, than in healthy subjects.
In patients with hepatic impairment of Child-Pugh A, B, and C, geometric mean AUCt of PAGN was 42%, 27%, and 22% lower, respectively, than that in healthy subjects.
The proportion of PBA excreted as PAGN in the urine in Child-Pugh A, B, and C was 80%, 58%, and 85%, respectively, and, in healthy volunteers, was 67%.
In another study in patients with hepatic impairment (Child-Pugh B and C), mean Cmax of PAA was 144 µg/mL (range: 14-358 µg/mL) after daily dosing of 6 mL of RAVICTI twice daily, while mean Cmax of PAA was 292 µg/mL (range: 57-655 µg/mL) after daily dosing of 9 mL of RAVICTI twice daily. The ratio of mean Cmax values for PAA to PAGN among all patients dosed with 6 mL and 9 mL twice daily were 3 and 3.7, respectively.
After multiple doses, a PAA concentration >200 µg/L was associated with a ratio of plasma PAA to PAGN concentrations higher than 2.5.
Renal Impairment
The pharmacokinetics of RAVICTI in patients with impaired renal function, including those with end-stage renal disease (ESRD) or those on hemodialysis, have not been studied.
Drug Interaction Studies
In vitro studies using human liver microsomes showed that the principle metabolite, phenylbutyrate, at a concentration of 800 µg/mL caused >60% reversible inhibition of cytochrome P450 isoenzymes CYP2C9, CYP2D6, and CYP3A4/5 (testosterone 6β-hydroxylase activity). Subsequent in vitro studies suggest that in vivo drug interactions with substrates of CYP2C9, CYP2D6, and CYP3A4/5 are possible. No in vivo drug interaction studies were conducted. The inhibition of CYP isoenzymes 1A2, 2C8, 2C19, and 2D6 by PAA at the concentration of 2.8 mg/mL was observed in vitro. Clinical implication of these results is unknown.
In vitro PBA or PAA did not induce CYP1A2 and CYP3A4, suggesting that in vivo drug interactions via induction of CYP1A2 and CYP3A4 are unlikely.
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, mutagenesis, impairment of fertility
Carcinogenesis
In a 2-year study in Sprague-Dawley rats, glycerol phenylbutyrate caused a statistically significant increase in the incidence of pancreatic acinar cell adenoma, carcinoma, and combined adenoma or carcinoma at a dose of 650 mg/kg/day in males (4.7 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) and 900 mg/kg/day in females (8.4 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA). The incidence of the following tumors was also increased in female rats at a dose of 900 mg/kg/day: thyroid follicular cell adenoma, carcinoma and combined adenoma or carcinoma, adrenal cortical combined adenoma or carcinoma, uterine endometrial stromal polyp, and combined polyp or sarcoma. The dose of 650 mg/kg/day in male rats is 3 times the dose of 7.45 mL/m2/day in pediatric patients, based on combined AUCs for PBA and PAA. The dose of 900 mg/kg/day in female rats is 5.5 times the dose of 7.45 mL/m2/day in pediatric patients, based on combined AUCs for PBA and PAA. In a 26-week study in transgenic (Tg.rasH2) mice, glycerol phenylbutyrate was not tumorigenic at doses up to 1000 mg/kg/day.
Mutagenesis
Glycerol phenylbutyrate was not genotoxic in the Ames test, the in vitro chromosomal aberration test in human peripheral blood lymphocytes, or the in vivo rat micronucleus test. The metabolites PBA, PAA, PAGN, and phenylacetylglycine were not genotoxic in the Ames test or in vitro chromosome aberration test in Chinese hamster ovary cells.
Impairment of Fertility
Glycerol phenylbutyrate had no effect on fertility or reproductive function in male and female rats at oral doses up to 900 mg/kg/day. At doses of 1200 mg/kg/day (approximately 7 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA), maternal toxicity was observed and the number of nonviable embryos was increased.
14 CLINICAL STUDIES
14.1 Clinical Studies in Adult Patients with UCDs
Active-Controlled, 4-Week, Noninferiority Study (Study 1)
A randomized, double-blind, active-controlled, crossover, noninferiority study (Study 1) compared RAVICTI to sodium phenylbutyrate by evaluating venous ammonia levels in patients with UCDs who had been on sodium phenylbutyrate prior to enrollment for control of their UCD. Patients were required to have a confirmed diagnosis of UCD involving deficiencies of CPS, OTC, or ASS, confirmed via enzymatic, biochemical, or genetic testing. Patients had to have no clinical evidence of hyperammonemia at enrollment and were not allowed to receive drugs known to increase ammonia levels (e.g., valproate), increase protein catabolism (e.g., corticosteroids), or significantly affect renal clearance (e.g., probenecid).
The primary endpoint was the 24-hour AUC (a measure of exposure to ammonia over 24 hours) for venous ammonia on days 14 and 28 when the drugs were expected to be at steady state. Statistical noninferiority would be established if the upper limit of the 2-sided 95% CI for the ratio of the geometric means (RAVICTI/sodium phenylbutyrate) for the endpoint was ≤ 1.25.
Forty-five patients were randomized 1:1 to 1 of 2 treatment arms to receive either
– Sodium phenylbutyrate for 2 weeks → RAVICTI for 2 weeks; or
– RAVICTI for 2 weeks → sodium phenylbutyrate for 2 weeks.
Sodium phenylbutyrate or RAVICTI were administered TID with meals. The dose of RAVICTI was calculated to deliver the same amount of PBA as the sodium phenylbutyrate dose the patients were taking when they entered the trial. Forty-four patients received at least 1 dose of RAVICTI in the trial.
Patients adhered to a low-protein diet and received amino acid supplements throughout the study. After 2 weeks of dosing, by which time patients had reached steady state on each treatment, all patients had 24 hours of ammonia measurements.
Demographic characteristics of the 45 patients enrolled in Study 1 were as follows: mean age at enrollment was 33 years (range: 18-75); 69% were female; 33% had adult-onset disease; 89% had OTC deficiency; 7% had ASS deficiency; 4% had CPS deficiency.
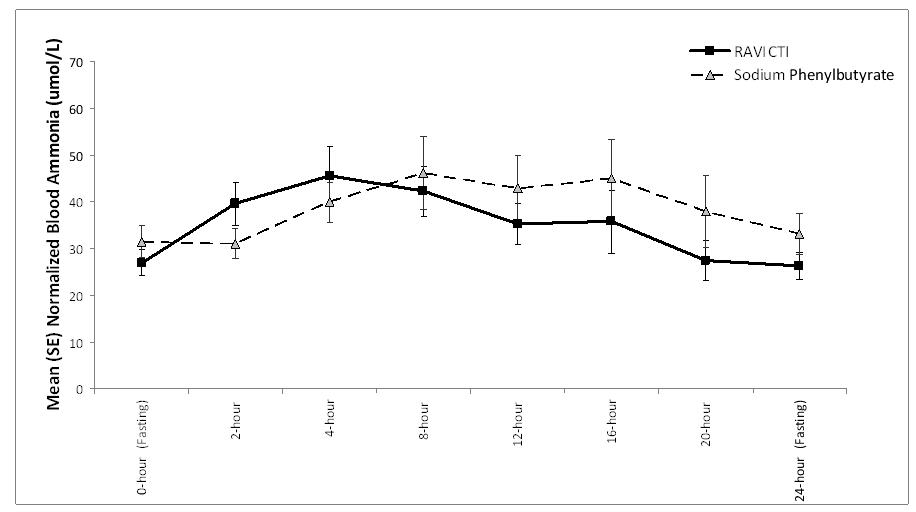
RAVICTI was noninferior to sodium phenylbutyrate with respect to the 24-hour AUC for ammonia. Forty-four patients were evaluated in this analysis. Mean 24-hour AUCs for venous ammonia during steady-state dosing were 866 µmol/L∙hour and 977 µmol/L∙hour with RAVICTI and sodium phenylbutyrate, respectively. The ratio of geometric means = 0.91 (95% CIs = 0.8-1.04).
The mean venous ammonia levels over 24-hours after 2 weeks of dosing (on day 14 and 28) in the double-blind short-term study (Study 1) are displayed in Figure 2 below. The mean and median maximum venous ammonia concentration (Cmax) over 24 hours and 24-hour AUC for venous ammonia are summarized in Table 2. Ammonia values across different laboratories were normalized to a common normal range of 9 to 35 µmol/L using the following formula after standardization of the units to µmol/L:
Normalized ammonia (µmol/L) = ammonia readout in µmol/L x (35/ULN of a laboratory reference range specified for each assay)
Figure 2: Venous Ammonia Response in Adult UCD Patients in Short-Term Treatment Study 1
| Timepoint | Ammonia (n=44) | ||||||||
| Mean (SD) | Median (min, max) | ||||||||
| Daily Cmax (µmol/L) | |||||||||
| RAVICTI | 61 (46) | 51 (12, 245) | |||||||
| Sodium phenylbutyrate | 71 (67) | 46 (14, 303) | |||||||
| 24-Hour AUC [(µmol/L)*hours] | |||||||||
| RAVICTI | 866 (661) | 673 (206,3351) | |||||||
| Sodium phenylbutyrate | 977 (865) | 653 (302, 4666) | |||||||
Open-Label Uncontrolled Extension Study in Adults
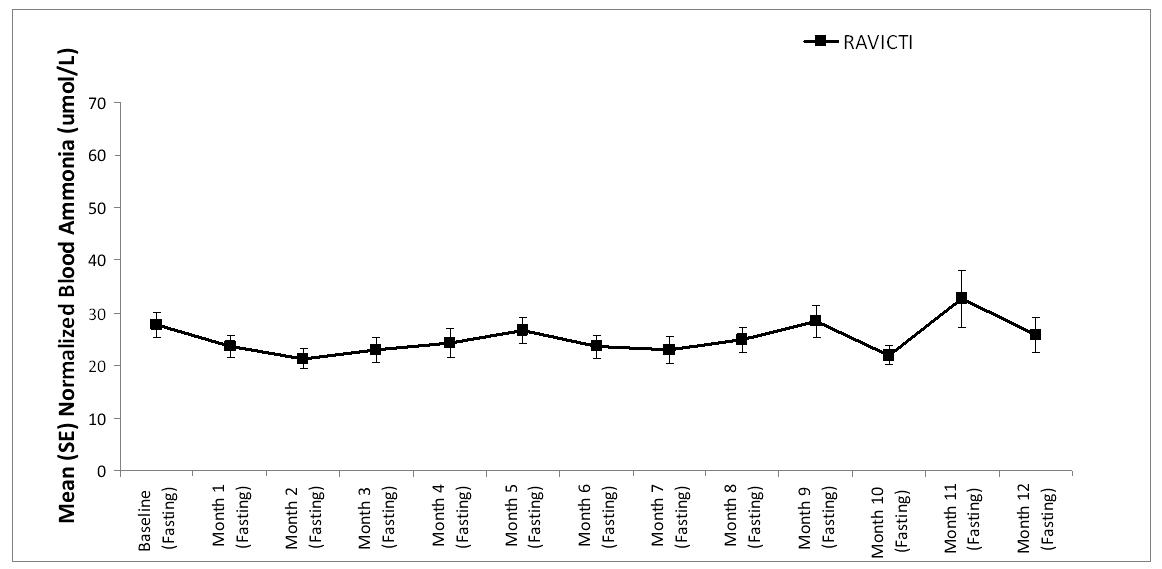
A long-term (12-month), uncontrolled, open-label study (Study 2) was conducted to assess monthly ammonia control and hyperammonemic crisis over a 12-month period. A total of 51 adults were in the study and all but 6 had been converted from sodium phenylbutyrate to RAVICTI. Venous ammonia levels were monitored monthly. Mean fasting venous ammonia values in adults in Study 2 were within normal limits during long-term treatment with RAVICTI (range: 6-30 µmol/L). Of 51 adult patients participating in the 12-month, open-label treatment with RAVICTI, 7 patients (14%) reported a total of 10 hyperammonemic crises. The fasting venous ammonia measured during Study 2 is displayed in Figure 3. Ammonia values across different laboratories were normalized to a common normal range of 9 to 35 µmol/L.
Figure 3: Venous Ammonia Response in Adult UCD Patients in Long-Term Treatment Study 2
14.2 Clinical Studies in Pediatric Patients With UCDs
The efficacy of RAVICTI in pediatric patients 2 to 17 years of age was evaluated in 2 fixed-sequence, open-label, sodium phenylbutyrate to RAVICTI switchover studies (Studies 3 and 4). Study 3 was 7 days in duration and Study 4 was 10 days in duration.
These studies compared blood ammonia levels of patients on RAVICTI to venous ammonia levels of patients on sodium phenylbutyrate in 26 pediatric UCD patients between 2 months and 17 years of age. Four patients <2 years of age are excluded for this analysis due to insufficient data. The dose of RAVICTI was calculated to deliver the same amount of PBA as the dose of sodium phenylbutyrate patients were taking when they entered the trial. Sodium phenylbutyrate or RAVICTI were administered in divided doses with meals. Patients adhered to a low-protein diet throughout the study. After a dosing period with each treatment, all patients underwent 24 hours of venous ammonia measurements, as well as blood and urine PK assessments.
UCD subtypes included OTC (n=12), argininosuccinate lyase (ASL) (n=8), and ASS deficiency (n=2), and patients received a mean RAVICTI dose of 7.9 mL/day (8 mL/m2/day, 8.8 g/m2/day), with doses ranging from 1.4 to 17.4 mL/day (1.5 to 14.4 g/m2/day). Doses in these patients were based on previous dosing of sodium phenylbutyrate.
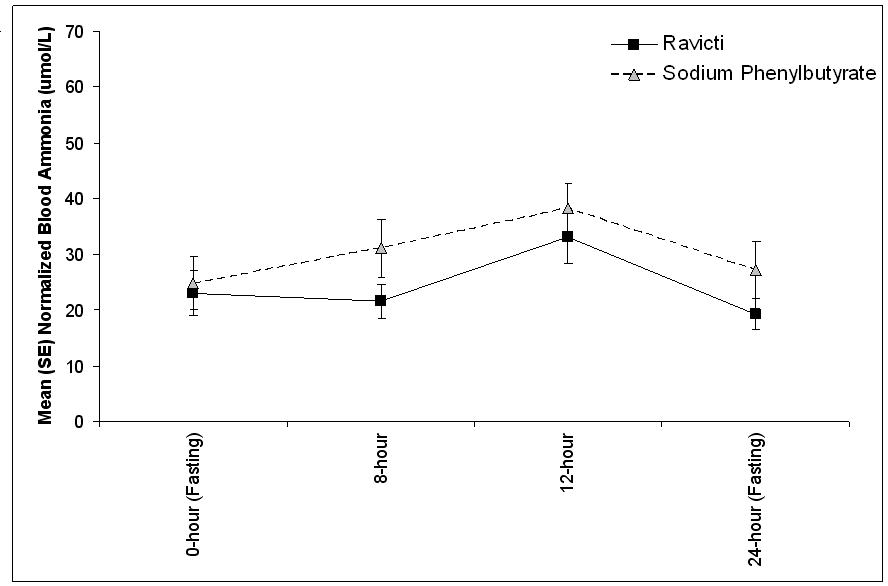
The 24-hour AUCs for blood ammonia (AUC0-24h) in 11 pediatric UCD patients 6 to 17 years of age (Study 3) and 11 pediatric UCD patients 2 years to 5 years of age (Study 4) were similar between treatments. In children 6 to 17 years of age, the ammonia AUC0-24h was 604 µmol∙h/L vs 815 µmol∙h/L on RAVICTI vs sodium phenylbutyrate. In the UCD patients between 2 years and 5 years of age, the ammonia AUC0-24h was 632 µmol∙h/L vs 720 µmol∙h/L on RAVICTI vs sodium phenylbutyrate.
The mean venous ammonia levels over 24 hours in open-label, short-term Studies 3 and 4 at common time points are displayed in Figure 4. Ammonia values across different laboratories were normalized to a common normal range of 9 to 35 µmol/L using the following formula after standardization of the units to µmol/L:
Normalized ammonia (µmol/L) = ammonia readout in µmol/L x (35/ULN of a laboratory reference range specified for each assay)
Figure 4: Venous Ammonia Response in Pediatric UCD Patients in Short-Term Treatment Studies 3 and 4
Open-Label, Uncontrolled, Extension Studies in Children
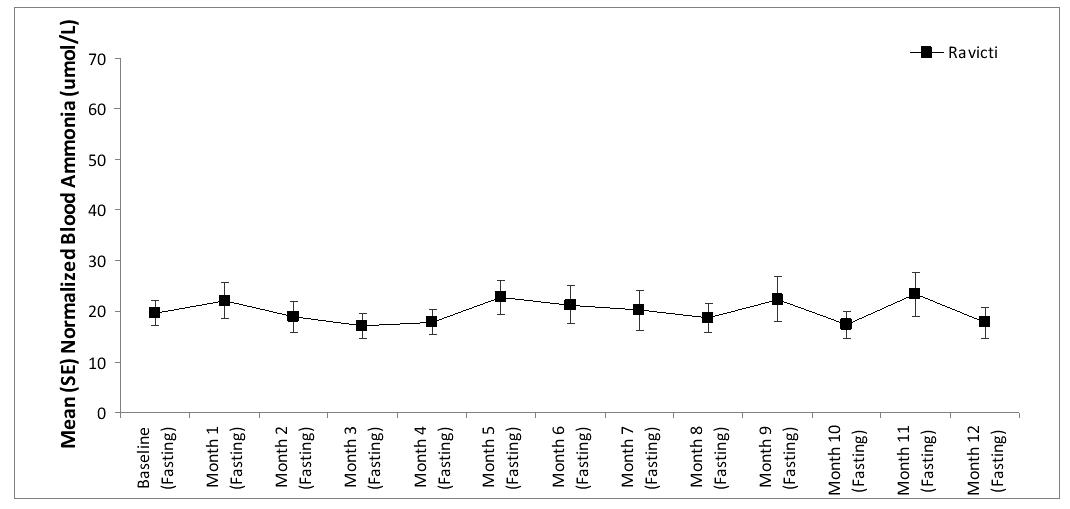
Long-term (12-month), uncontrolled, open-label study were conducted to assess monthly ammonia control and hyperammonemic crisis over a 12-month period. In two studies (Study 2, which also enrolled adults, and an extension of Study 3, referred to here as Study 3E), a total of 26 children ages 6 to 17, were enrolled, and all but 1 had been converted from sodium phenylbutyrate to RAVICTI. Mean fasting venous ammonia values were within normal limits during long-term treatment with RAVICTI (range: 17-23 µmol/L). Of the 26 pediatric patients 6 to 17 years of age participating in these two trials, 5 patients (19%) reported a total of 5 hyperammonemic crises. The fasting venous ammonia measured during these two extension studies in patients 6 to 17 years is displayed in Figure 5. Ammonia values across different laboratories were normalized to a common normal range of 9 to 35 µmol/L.
Figure 5: Venous Ammonia Response in Pediatric UCD Patients in Long-Term Treatment Studies 2 and 3E
In an extension of Study 4, after a median time on study of 4.5 months (range 1.0-5.7 months), 2 of 16 pediatric patients ages 2 to 5 years had experienced three hyperammonemic crises.
15. REFERENCES
1. Brusilow SW. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr Res. 1991;29(2):147-150.
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide).
Instruct patients or their caregivers on the following information necessary for patients to use the drug safely and effectively:
Instruct patients that the risks associated with RAVICTI include the following:
Common adverse reactions of RAVICTI include diarrhea, gas, and headache. Adverse reactions of RAVICTI are sometimes the same as symptoms of high blood ammonia. Headache, feeling tired, lightheadedness, and confusion are possible adverse reactions of RAVICTI. If the patient experiences these symptoms, instruct the patient to call their doctor right away. The major metabolite of RAVICTI, PAA, is associated with neurological toxicity manifested by somnolence, fatigue, lightheadedness, headache, dysgeusia, hypoacusis, disorientation, and impaired memory. These symptoms of neurological toxicity may be reversible. Instruct the patient to call their doctor if exhibiting these symptoms. Blood tests may be done to measure the amount of PAA in the blood.
RAVICTI Registry
Patients and their caregivers should be informed that a registry for UCD patients has been established by Hyperion Therapeutics in order to better assess long-term outcomes in patients with UCDs, including growth and neurocognitive outcomes and the outcome of pregnancy for women with UCDs who become pregnant. Patients and their caregivers are encouraged to participate in the registry and advised that their participation is voluntary. For more information regarding the registry program, call 1-855-823-2595.
Manufactured by:
Lyne Laboratories, Inc.
Brockton, MA 02301
Manufactured for:
Hyperion Therapeutics Inc.
601 Gateway Boulevard, Suite 200
South San Francisco, CA 94080
© Hyperion Therapeutics, Inc.
All rights reserved.
RAVICTI is a trademark of Hyperion Therapeutics, Inc.
MEDICATION GUIDE
RAVICTI™ (rah-VIK- tee)
(glycerol phenylbutyrate)
oral liquid
What is the most important information I should know about RAVICTI?
RAVICTI may cause serious side effects, including:
Nervous system problems (Neurotoxicity). Phenylacetate, a breakdown product of RAVICTI, may cause nervous system side effects. Call your doctor or get medical help right away if you get any of these symptoms while taking RAVICTI:
- sleepiness
- weakness
- lightheadedness
- change in taste
- problems with hearing
- confusion
- problems with memory
- worsening neuropathy (numbness, tingling, or burning in your hands or feet)
- headache
What is RAVICTI?
RAVICTI is a prescription medicine used in adults and children 2 years of age and older for long-term management of high blood levels of ammonia (hyperammonemia) caused by a condition called urea cycle disorder (UCD). Ravicti should be used if the UCD cannot be managed with a low protein diet and dietary supplements alone. RAVICTI must be used along with a low protein diet and in some cases dietary supplements.
RAVICTI is not used for the treatment of hyperammonemia in people with UCD.
It is not known if RAVICTI is safe and effective for the treatment of N-acetyglutamate synthase (NAGS) deficiency.
It is not known if RAVICTI is safe and effective in children 2 months to less than 2 years of age.
Who should not take RAVICTI?
- Children less than 2 months of age should not take RAVICTI because it may not be digested in babies less than 2 months of age.
- Do not take RAVICTI if you are allergic to phenylbutyrate.
Call your doctor or go to the nearest hospital emergency room if you get wheezing, shortness of breath, cough, low blood pressure, flushing, nausea or a rash while taking RAVICTI.
What should I tell my doctor before taking RAVICTI?
Before you take RAVICTI, tell your doctor if you:
- have liver or kidney problems
- have pancreas or bowel (intestine) problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if RAVICTI will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if RAVICTI passes into your breast milk. RAVICTI may harm your baby, so you and your doctor should decide if you will take RAVICTI or breastfeed.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, dietary and herbal supplements.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take RAVICTI?
- Take RAVICTI exactly as your doctor tells you.
- Your doctor will tell you how much RAVICTI to take and when to take it.
- Your doctor may change your dose if needed.
- Take RAVICTI with food.
- RAVICTI is an oral liquid that is taken by mouth using an oral syringe or measuring cup. Ask your pharmacist for an oral syringe or measuring cup if you do not have one.
- Stay on the diet that your doctor gives you.
- If you take too much RAVICTI, call your doctor or go to the nearest hospital emergency room right away.
- Talk to your doctor about participating in a UCD registry. The purpose of this registry is to collect information about people with UCD to improve care. For more information about the registry program call 1-855-823-2595 or visit www.ucdregistry.com.
For people who have a nasogastric or gastric tube in place, RAVICTI should be given as follows:
- Use an oral syringe to withdraw the prescribed dose of RAVICTI from the bottle.
- Place the tip of the syringe into the tip of the nasogastric or gastric tube and push the plunger of the syringe to give RAVICTI into the tube.
- Flush the nasogastric or gastric tube with 30 mL of water and allow the flush to drain.
- Flush the nasogastric or gastric tube a second time with an additional 30 mL of water.
What are the possible side effects of RAVICTI?
RAVICTI may cause serious side effects, including:
- See "What is the most important information I should know about RAVICTI?"
- The most common side effects of RAVICTI in adults include:
- diarrhea
- gas
- headache
- nausea
- vomiting
- tiredness
- decrease appetite
- high blood levels of ammonia
- dizziness
The most common side effect of RAVICTI in children include:
- upper abdomen (stomach) pain
- nausea
- vomiting
- diarrhea
- decreased appetite
- high blood levels of ammonia
- headache
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of RAVICTI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store RAVICTI?
- Store RAVICTI between 68°F to 77°F (20°C to 25°C).
Keep RAVICTI and all medicines out of the reach of children.
General information about the safe and effective use of RAVICTI
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use RAVICTI for a condition for which it was not prescribed. Do not give RAVICTI to other people, even if they have the same symptoms you have. It may harm them.
If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about RAVICTI that is written for health professionals.
For more information call 1-855-823-7878 or go to www.RAVICTI.com.
What are the ingredients in RAVICTI?
Active ingredient: glycerol phenylbutyrate
This Medication Guide has been approved by the U.S. Food and Drug
Administration.
Manufactured by:
Lyne Laboratories, Inc.
Brockton, MA 02301
Manufactured for:
Hyperion Therapeutics Inc.
601 Gateway Boulevard, Suite 200
South San Francisco, CA 94080
Issued: February 2013
© Hyperion Therapeutics, Inc.
All rights reserved.
RAVICTI is a trademark of Hyperion Therapeutics, Inc.
| RAVICTI
glycerol phenylbutyrate liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Hyperion Therapeutics, Inc. (807130500) |