TEMOVATE E- clobetasol propionate cream
Fougera Pharmaceuticals Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TEMOVATE® E safely and effectively. See full prescribing information for TEMOVATE® E.
TEMOVATE ® E (clobetasol propionate) cream, 0.05%, for topical use Initial U.S. Approval: 1994 INDICATIONS AND USAGETemovate® E is a corticosteroid indicated for:
1.3 Limitation of Use DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSCream: 0.05% (3) CONTRAINDICATIONSNone (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact PharmaDerm, A division of Fougera Pharmaceuticals Inc. at 1-800-645-9833 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION. Revised: 7/2021 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Corticosteroid-Responsive Dermatoses
Temovate® E is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients 12 years of age and older. Treatment should be limited to 2 consecutive weeks, and the total dosage should not exceed 50 grams per week
2 DOSAGE AND ADMINISTRATION
- Apply a thin layer of Temovate® E to the affected skin areas twice daily and rub in gently and completely. Wash hands after each application.
Temovate® E is a super-high potency topical corticosteroid; therefore, treatment should be limited to 2 consecutive weeks, and amounts greater than 50 grams per week should not be used.
- In moderate to severe plaque-type psoriasis, Temovate® E applied to 5% to 10% of body surface area can be used for up to 4 weeks. The total dosage should not exceed 50 grams per week. When dosing for more than 2 weeks, any additional benefits of extending treatment should be weighed against the risk of HPA suppression. Therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. Treatment beyond 4 consecutive weeks is not recommended.
Temovate® E should not be used with occlusive dressings.
3 DOSAGE FORMS AND STRENGTHS
Cream, 0.05%. Each gram of Temovate® E Cream contains 0.5 mg of clobetasol propionate in a white to off-white cream base.
5 WARNINGS AND PRECAUTIONS
5.1 Effects on the Endocrine System
- Clobetasol propionate is a highly potent topical corticosteroid that has been shown to suppress the HPA axis at doses as low as 2 grams per day.
Systemic absorption of topical corticosteroids can produce reversible HPA axis suppression with the potential for clinical glucocorticosteroid insufficiency This may occur during treatment or upon withdrawal of the topical corticosteroid. Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. In a study including 12 subjects ages 18 years and older with psoriasis or atopic dermatitis involving at least 30% body surface area (BSA), adrenal suppression was identified in 3 out of 12 subjects (25%) following 1 week of treatment.
Factors that predispose a patient using a topical corticosteroid to HPA axis suppression include the use of more potent steroids, use over large surface areas use over prolonged periods, use under occlusion, use on an altered skin barrier, and use in patients with liver failure.
- An ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression. If HPA axis suppression is documented, an attempt should be made to gradually withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Manifestations of adrenal insufficiency may require supplemental systemic corticosteroids. Recovery of HPA axis function is generally prompt and complete upon discontinuation of topical corticosteroids.
Cushing's syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids.
Use of more than one corticosteroid-containing product at the same time may increase the total systemic corticosteroid exposure.
- Pediatric patients may be more susceptible to systemic toxicity from use of topical corticosteroids. [ see Use in Specific Populations (8.4) ]
5.2 Local Adverse Reactions with Topical Corticosteroids
- Local adverse reactions may be more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids. Reactions may include atrophy, striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, hypertrichosis, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria. Some local adverse reactions may be irreversible. Clobetasol propionate is not recommended in patients with acne vulgaris, rosacea or perioral dermatitis.
5.3 Allergic Contact Dermatitis
Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noting a clinical exacerbation. Clinical diagnosis of allergic contact dermatitis can be confirmed with patch testing. If irritation develops, Temovate® E should be discontinued and appropriate therapy instituted.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In controlled trials with clobetasol propionate formulations, the following adverse reactions have been reported:burning/stinging, pruritis, irritation, erythema, folliculitis, cracking and fissuring of the skin, numbness of the fingers tenderness in the elbow, skin atrophy, and telangiectasia. The incidence of local adverse reactions reported in the trials with Temovate® E was less than 2% of patients treated with the exception of burning/stinging which occurred in 5% of treated patients.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
- Pregnancy Category C.
- There are no adequate and well-controlled studies in pregnant women. Therefore, Temovate ® E should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application to laboratory animals. Clobetasol propionate has not been tested for teratogenicity by this route; however, it is absorbed percutaneously, and when administered subcutaneously it was a significant teratogen in both the rabbit and mouse Clobetasol propionate has greater teratogenic potential than steroids that are less potent.
- Teratogenicity studies in mice using the subcutaneous route resulted in fetotoxicity at the highest dose tested (1 mg/kg) and teratogenicity at all dose levels tested down to 0.03 mg/kg. These doses are approximately 0.33 and 0.01 times, respectivelythe human topical dose of Temovate® E. Abnormalities seen included cleft palate and skeletal abnormalities.
In rabbits, clobetasol propionate was teratogenic at doses of 3 and 10 mcg/kg. These doses are approximately 0.001 and 0.003 times, respectively, the human topical dose of Temovate® E. Abnormalities seen included cleft palate, cranioschisis, and other skeletal abnormalities.
8.3 Nursing Mothers
- Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when Temovate ® E is administered to a nursing woman.
8.4 Pediatric Use
and effectiveness of Temovate® E in pediatric patients have not been established and its use in pediatric patients under years of age is not recommended. In a study including 12 subjects ages 18 years and older with psoriasis or atopic dermatitis involving at least 30% body surface area (BSA), adrenal suppression was identified in 3 out of 12 subjects (25%) following 1 week of treatment. Four-week HPA axis suppression studies with Temovate® E Cream in pediatric subjects have not been conducted.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing's syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of glucocorticosteroid insufficiency during or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children.
HPA axis suppression, Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
- The use of Temovate® E for 4 consecutive weeks has not been studied in pediatric patients under 16 years of age.
10 OVERDOSAGE
Topically applied Temovate® E can be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5.1)].
11 DESCRIPTION
Temovate® E (clobetasol propionate) Cream, 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity.
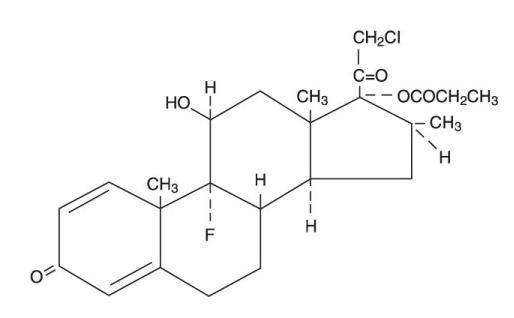
Chemically, clobetasol propionate is (11β,16β)-21-chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)-pregna-1,4-diene-3,20-dione, and it has the following structural formula:
- Clobetasol propionate has the molecular formula C25H32ClFO5 and a molecular weight of 467. It is a white to cream-colored crystalline powder insoluble in water.
- Each gram of Temovate® E contains 0.5 mg of clobetasol propionate in a white to off-white cream base consisting of cetostearyl alcohol, isopropyl myristate, propylene glycol, cetomacrogol 1000, dimethicone 360, citric acid, sodium citrate, purified water, and imidurea as a preservative.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
- Like other topical corticosteroids, clobetasol propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2 .
12.2 Pharmacodynamics
- Temovate® E is in the super-high range of potency as demonstrated in a vasoconstrictor study in healthy subjects when compared with other topical corticosteroids. However, similar blanching scores do not necessarily imply therapeutic equivalence.
12.3 Pharmacokinetics
- The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies have not been performed to evaluate the carcinogenic potential of clobetasol propionate. Mutagenesis
Clobetasol propionate was nonmutagenic in three different test systems: the Ames test, the Saccharomyces cerevisiae gene conversion assay, and the E. Coli B WP2 fluctuation test.
Impairment of Fertility
Studies in the rat following oral administration at dosage levels up to 50 mg/kg per day revealed no significant effect on the males. The females exhibited an increase in the number of resorbed embryos and a decrease in the number of living fetuses at the highest dose.
14 CLINICAL STUDIES
In a controlled clinical trial involving patients with moderate to severe plaque-type psoriasis, Temovate® E was applied to 5% to 10% of body surface area. In this trial, there were no clobetasol-treated patients with clinically significant decreases in morning cortisol levels after 4 weeks of treatment; however, morning cortisol levels may not identify patients with adrenal dysfunction.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Temovate® E (clobetasol propionate) Cream, 0.05% is a white to off-white cream, supplied in 60 g tubes (NDC 10337-301-60).
17 PATIENT COUNSELING INFORMATION
Inform patients using topical corticosteroids of the following information and instructions:
- •
- Temovate® E is for external use only. Avoid contact with the eyes.
- •
- Use as directed. Do not use Temovate® E for any disorder other than that for which it was prescribed. Do not use longer than the prescribed time period.
- •
- Do not use other corticosteroid-containing products while using Temovate® E unless directed by the physician. The treated skin area should not be bandaged, otherwise covered, or wrapped so as to be occlusive unless directed by the physician.
- •
- Wash hands after applying the medication.
- •
- Report any signs of local or systemic adverse reactions to the physician.
- •
- Inform their physicians that they are using Temovate® E if surgery is contemplated. If you go to another doctor for illness, injury or surgery, tell the doctor you are using Temovate® E.
- •
- Do not use Temovate® E on the face, underarms or groin areas. As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, contact the physician
- •
- Use no more than 50 grams per week of Temovate® E
- •
- Store between 59°F and 86° (15°C and 30°C). Do not refrigerate.
PharmaDerm®
a division of
Fougera
PHARMACEUTICALS INC.
Melville, NY 11747
www.pharmaderm.com
I8301E
R11/2014
#154
| TEMOVATE E
clobetasol propionate cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Fougera Pharmaceuticals Inc. (043838424) |