Label: CHLORAPREP ONE-STEP- chlorhexidine gluconate and isopropyl alcohol solution
- NDC Code(s): 54365-400-04, 54365-400-09, 54365-400-12
- Packager: CareFusion 213 LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 14, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purposes
- Use
-
Warnings
For external use only. Flammable, keep away from fire or flame. To reduce the risk of fire, PREP CAREFULLY:
- solution contains alcohol and gives off flammable vapors
- avoid getting solution into hairy areas. Hair may take up to 1 hour to dry. Wet hair is flammable.
- do not drape or use ignition source (e.g., cautery, laser) until solution is completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair)
- do not allow solution to pool
- remove wet materials from prep area
Allergy alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do not use
- on patients with known allergies to chlorhexidine gluconate or any other ingredient in this product.
- for lumbar puncture or in contact with the meninges
- on open skin wounds or as a general skin cleanser
When using this product
keep out of eyes, ears, and mouth. May cause serious or permanent injury if permitted to enter and remain. If contact occurs, rinse with cold water right away and contact a doctor.
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- use in a well ventilated area
- maximal treatment area for one applicator is approximately 8.4 in. × 8.4 in. (457 cm 2)
- remove applicator from package; do not touch sponge
- hold the applicator with the sponge down. Pinch wings only once to activate the ampule and release the antiseptic.
- wet the sponge by pressing and releasing the sponge against the treatment area until liquid is visible on the skin
- completely wet the treatment area with antiseptic
- dry surgical sites (e.g., abdomen or arm): use gentle repeated back-and-forth strokes for 30 seconds
- moist surgical sites (e.g., inguinal fold): use gentle repeated back-and-forth strokes for 2 minutes
- do not allow solution to pool; tuck prep towels to absorb solution, and then remove
- allow the solution to completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair). Do not blot or wipe away.
- discard the applicator after a single use along with any portion of the solution not required to cover the prep area. It is not necessary to use the entire amount available.
- Other information
- Inactive ingredients
- Questions?
-
Package/Label Principal Display Panel 10.5mL Applicators Non-Sterile Solution: Clear, Hi-Lite Orange, Scrub Teal
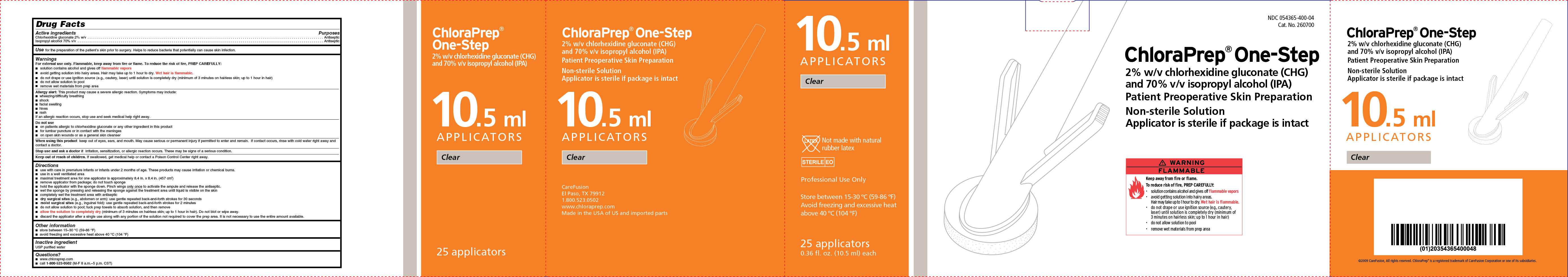
PRINCIPAL DISPLAY PANEL-CARTON
10.5 ml
APPLICATORS
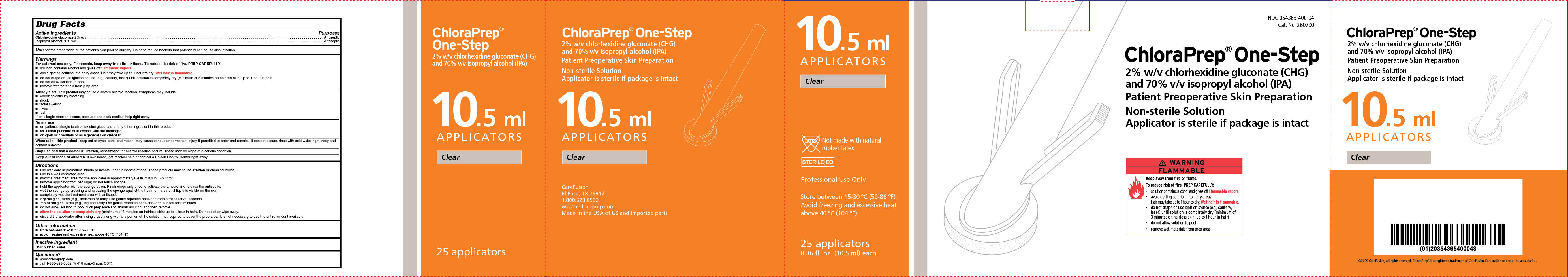
Clear
Not made with natural rubber latex
STERILE EO
Professional Use Only
Store between 15-30 °C (59-86 °F)
Avoid freezing and excessive heat
above 40 °C (104 °F)
25 applicators
0.36 fl. oz. (10.5 ml) each
NDC 054365-400-04
Cat. No. 260700
ChloraPrep® One-Step
2% w/v chlorhexidine gluconate (CHG)
and 70% isopropyl alcohol (IPA)
Patient Preoperative Skin Preparation
Non-sterile Solution
Applicator is sterile if package is intact
WARNING FLAMMABLE
Keep away from fire or flame.
To reduce risk of fire, PREP CAREFULLY:
- solution contains alcohol and gives off flammable vapors
- avoid getting solution into hairy areas.
Hair may take up to 1 hour to dry. Wet hair is flammable.
- do not drape or use ignition source (e.g., cautery,
laser) until solution is completely dry (minimum of
3 minutes on hairless skin; up to 1 hour in hair)
- do not allow solution to pool
- remove wet material from prep area
PRINCIPAL DISPLAY PANEL-CARTON
10.5 ml
APPLICATORS
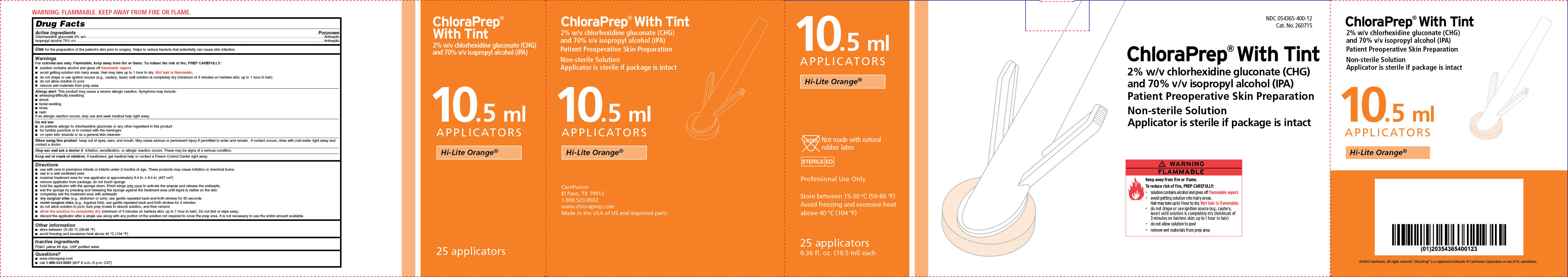
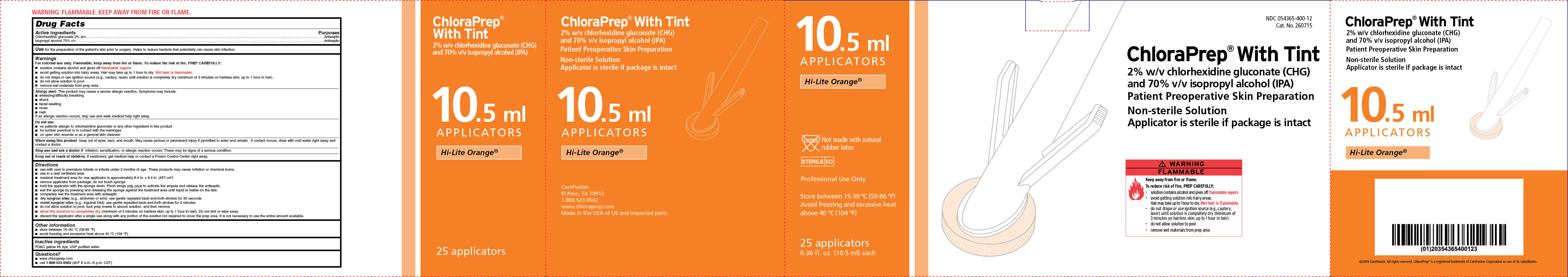
Hi-Lite Orange
Not made with natural rubber latex
STERILE EO
Professional Use Only
Store between 15-30 °C (59-86 °F)
Avoid freezing and excessive heat
above 40 °C (104 °F)
25 applicators
0.36 fl. oz. (10.5 ml) each
NDC 054365-400-12
Cat. No. 260715
ChloraPrep ® With Tint
2% w/v chlorhexidine gluconate (CHG)
and 70% isopropyl alcohol (IPA)
Patient Preoperative Skin Preparation
Non-sterile Solution
Applicator is sterile if package is intact
WARNING FLAMMABLE
Keep away from fire or flame.
To reduce risk of fire, PREP CAREFULLY:
- solution contains alcohol and gives off flammable vapors
- avoid getting solution into hairy areas.
Hair may take up to 1 hour to dry. Wet hair is flammable.
- do not drape or use ignition source (e.g., cautery,
laser) until solution is completely dry (minimum of
3 minutes on hairless skin; up to 1 hour in hair)
- do not allow solution to pool
- remove wet material from prep area
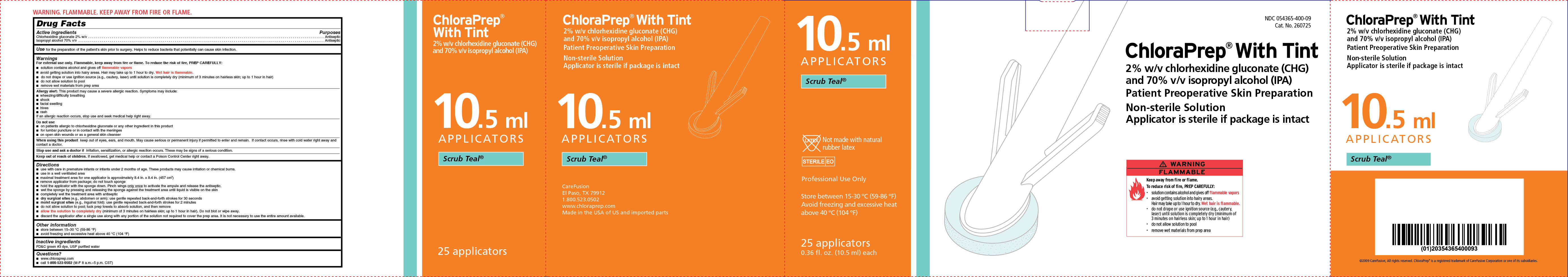
PRINCIPAL DISPLAY PANEL-CARTON
10.5 ml
APPLICATORS
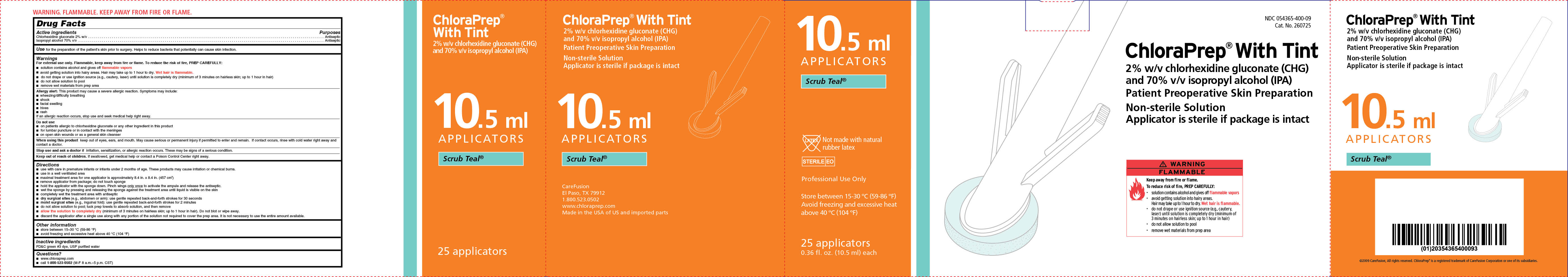
Scrub Teal
Not made with natural rubber latex
STERILE EO
Professional Use Only
Store between 15-30 °C (59-86 °F)
Avoid freezing and excessive heat
above 40 °C (104 °F)
25 applicators
0.36 fl. oz. (10.5 ml) each
NDC 054365-400-09
Cat. No. 260725
ChloraPrep® With Tint
2% w/v chlorhexidine gluconate (CHG)
and 70% isopropyl alcohol (IPA)
Patient Preoperative Skin Preparation
Non-sterile Solution
Applicator is sterile if package is intact
WARNING FLAMMABLE
Keep away from fire or flame.
To reduce risk of fire, PREP CAREFULLY:
- solution contains alcohol and gives off flammable vapors
- avoid getting solution into hairy areas.
Hair may take up to 1 hour to dry. Wet hair is flammable.
- do not drape or use ignition source (e.g., cautery,
laser) until solution is completely dry (minimum of
3 minutes on hairless skin; up to 1 hour in hair)
- do not allow solution to pool
- remove wet material from prep area
-
INGREDIENTS AND APPEARANCE
CHLORAPREP ONE-STEP
chlorhexidine gluconate and isopropyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54365-400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54365-400-04 25 in 1 CARTON 07/14/2000 1 1 in 1 POUCH 1 10.5 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:54365-400-12 25 in 1 CARTON 08/18/2006 2 1 in 1 POUCH 2 10.5 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 3 NDC:54365-400-09 25 in 1 CARTON 04/26/2002 3 1 in 1 POUCH 3 10.5 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020832 07/14/2000 Labeler - CareFusion 213 LLC (826496312) Registrant - Becton, Dickinson and Company (832696038) Establishment Name Address ID/FEI Business Operations CareFusion 213 LLC 826496312 analysis(54365-400) , manufacture(54365-400) , label(54365-400) , pack(54365-400) , sterilize(54365-400)