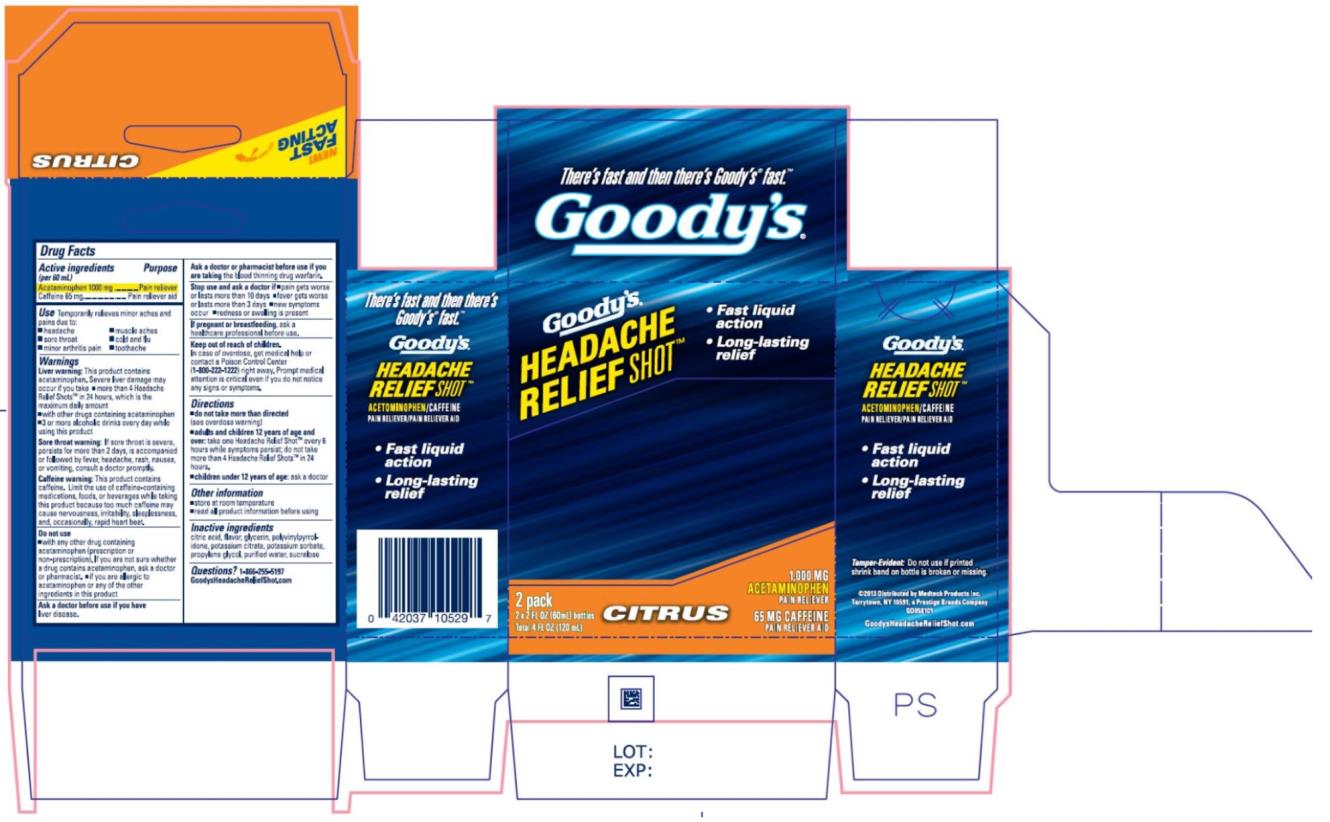
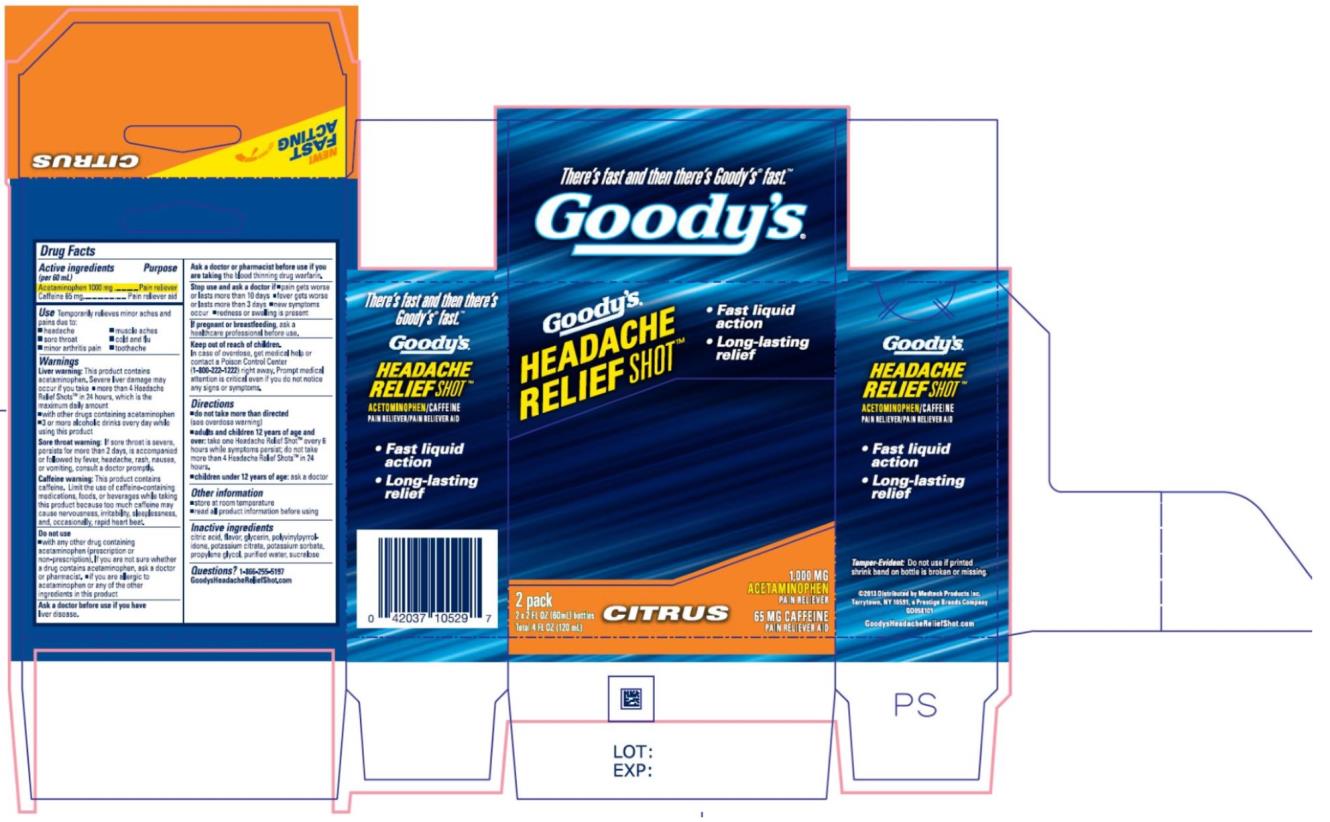
Label: GOODYS HEADACHE RELIEF SHOT- acetaminophen and caffeine liquid
- NDC Code(s): 63029-629-01, 63029-629-02, 63029-639-01, 63029-639-02
- Packager: Medtech Products Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 26, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
you take
• more than 4 Headache Relief Shots™ in 24 hours, which is the maximum daily amount
• with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this product
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Caffeine warning: This product contains caffeine. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
Do not use
• with any other drug containing acetaminophen (prescription or non-prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
• if you are allergic to acetaminophen or any of the inactive ingredients in this product
- Directions
- Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOODYS HEADACHE RELIEF SHOT
acetaminophen and caffeine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63029-639 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 1000 mg in 60 mL CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg in 60 mL Inactive Ingredients Ingredient Name Strength POVIDONE K30 (UNII: U725QWY32X) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM CITRATE (UNII: EE90ONI6FF) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color WHITE Score Shape Size Flavor CITRUS Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63029-639-01 2 in 1 DOSE PACK 02/11/2013 06/30/2023 1 60 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC:63029-639-02 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/11/2013 06/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 02/11/2013 06/30/2023 GOODYS HEADACHE RELIEF SHOT
acetaminophen and caffeine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63029-629 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 1000 mg in 60 mL CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg in 60 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM CITRATE (UNII: EE90ONI6FF) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) POVIDONE K30 (UNII: U725QWY32X) Product Characteristics Color WHITE Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63029-629-01 2 in 1 DOSE PACK 02/11/2013 1 60 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC:63029-629-02 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/11/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 02/11/2013 Labeler - Medtech Products Inc. (122715688)