CLONAZEPAM- clonazepam tablet
St Marys Medical Park Pharmacy
----------
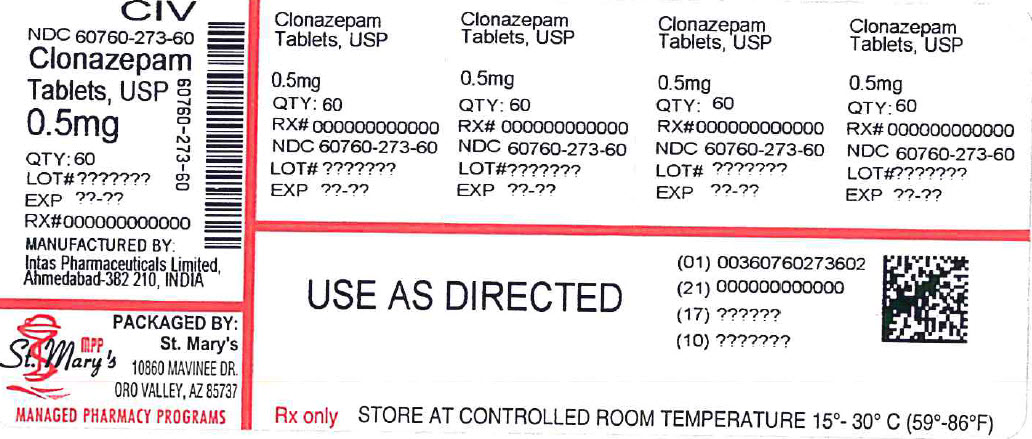
Clonazepam Tablets, USP
CIV
Rx only,
CONTRAINDICATIONS
Clonazepam should not be used in patients with a history of sensitivity to benzodiazepines, nor in patients with clinical or biochemical evidence of significant liver disease. It may be used in patients with open angle glaucoma who are receiving appropriate therapy but is contraindicated in acute narrow angle glaucoma.
ADVERSE REACTIONS
The adverse experiences for clonazepam are provided separately for patients with seizure disorders and with panic disorder.
DOSAGE AND ADMINISTRATION
Clonazepam is available as a tablet. The tablets should be administered with water by swallowing the tablet whole.
HOW SUPPLIED
Clonazepam tablets USP 0.5 mg are orange, round, flat faced, beveled edge, scored, debossed with “1” and “2” on one side and plain on other. They are supplied as follows:
NDC: 60760-273-60 BOTTLES OF 60
Clonazepam tablets USP 1 mg are blue, round, flat faced, beveled edge, debossed with “C 1” on one side and plain on the other. They are supplied as follows:
NDC: 60760-300-30 bottle of 30
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature].
Manufactured For:
Accord Healthcare, Inc.,
1009, Slater Road,
Suite 210-B,
Durham, NC 27703,
USA.
Manufactured By:
Intas Pharmaceuticals Limited,
Plot No. : 457, 458,
Village – Matoda,
Bavla Road, Ta.- Sanand,
Dist.- Ahmedabad – 382 210.
India.
10 7744 1 626035
Issued October, 2010.
Medication Guide
Clonazepam Tablets, USP
CIV
Read this Medication Guide before you start taking clonazepam tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Clonazepam tablets can cause serious side effects. Because stopping clonazepam tablets suddenly can also cause serious problems, do not stop taking clonazepam tablets without talking to your healthcare provider first.
What is the most important information I should know about clonazepam tablets?
Do not stop taking clonazepam tablets without first talking to your healthcare provider.
Stopping clonazepam tablets suddenly can cause serious problems.
Clonazepam tablets can cause serious side effects, including:
-
Clonazepam tablets can slow your thinking and motor skills
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how clonazepam tablets affects you.
- Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking clonazepam until you talk to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, clonazepam tablets may make your sleepiness or dizziness worse.
-
Like other antiepileptic drugs, clonazepam tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempt to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
Do not stop clonazepam tablets without first talking to a healthcare provider.
Stopping clonazepam tablets suddenly can cause serious problems. Stopping clonazepam tablets suddenly can cause seizures that will not stop (status epilepticus).
-
Clonazepam tablets may harm your unborn or developing baby.
- If you take clonazepam tablets during pregnancy, your baby is at risk for serious birth defects. These defects can happen as early as in the first month of pregnancy, even before you know you are pregnant. Birth defects may occur even in children born to women who are not taking any medicines and do not have other risk factors.
- Children born to mothers receiving benzodiazepine medications (including clonazepam tablets) late in pregnancy may be at some risk of experiencing breathing problems, feeding problems, hypothermia, and withdrawal symptoms.
- Tell your healthcare provider right away if you become pregnant while taking clonazepam tablets. You and your healthcare provider should decide if you will take clonazepam tablets while you are pregnant.
- If you become pregnant while taking clonazepam tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can register by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- Clonazepam tablets can pass into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take clonazepam tablets. You and your healthcare provider should decide if you will take clonazepam tablets or breast feed. You should not do both.
-
Clonazepam tablets can cause abuse and dependence.
- Do not stop taking clonazepam tablets all of a sudden. Stopping clonazepam tablets suddenly can cause seizures that do not stop, hearing or seeing things that are not there (hallucinations), shaking, and stomach and muscle cramps.
- Talk to your doctor about slowly stopping clonazepam tablets to avoid getting sick with withdrawal symptoms.
- Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- Do not stop taking clonazepam tablets all of a sudden. Stopping clonazepam tablets suddenly can cause seizures that do not stop, hearing or seeing things that are not there (hallucinations), shaking, and stomach and muscle cramps.
Clonazepam tablets are a federally controlled substance (C-IV) because it can be abused or lead to dependence. Keep clonazepam tablets in a safe place to prevent misuse and abuse. Selling or giving away clonazepam tablets may harm others, and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
What is a clonazepam tablet?
Clonazepam tablet is a prescription medicine used alone or with other medicines to treat:
- certain types of seizure disorders (epilepsy) in adults and children
- panic disorder with or without fear of open spaces (agoraphobia) in adults
It is not known if clonazepam tablet is safe or effective in treating panic disorder in children younger than 18 years old.
Who should not take clonazepam tablets?
Do not take clonazepam tablets if you:
- are allergic to benzodiazepines
- have significant liver disease
- have an eye disease called acute narrow angle glaucoma
Ask your healthcare provider if you are not sure if you have any of the problems listed above.
What should I tell my healthcare provider before taking clonazepam tablets?
Before you take clonazepam tablets, tell your healthcare provider if you:
- have liver or kidney problems
- have lung problems (respiratory disease)
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have any other medical conditions
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Taking clonazepam tablets with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take clonazepam tablets?
- Take clonazepam tablets exactly as your healthcare provider tells you. Clonazepam is available as a tablet.
- Do not stop taking clonazepam tablets without first talking to your healthcare provider.
Stopping clonazepam tablets suddenly can cause serious problems.
- Clonazepam tablets should be taken with water and swallowed whole.
- If you take too much clonazepam tablets, call your healthcare provider or local Poison Control Center right away.
What should I avoid while taking clonazepam tablets?
- Clonazepam tablets can slow your thinking and motor skills. Do not drive, operate heavy machinery, or do other dangerous activities until you know how clonazepam tablets affects you.
- Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking clonazepam until you talk to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, clonazepam tablets may make your sleepiness or dizziness worse.
What are the possible side effects of clonazepam tablets?
See “What is the most important information I should know about clonazepam tablets?”
Clonazepam tablets can also make your seizures happen more often or make them worse. Call your healthcare provider right away if your seizures get worse while taking clonazepam tablets.
The most common side effects of clonazepam tablets include:
- Drowsiness
- Problems with walking and coordination
- Dizziness
- Depression
- Fatigue
- Problems with memory
These are not all the possible side effects of clonazepam tablets. For more information, ask your healthcare provider or pharmacist.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store clonazepam tablets?
- Store clonazepam tablets between 59ºF to 86ºF (15ºC to 30ºC).
Keep clonazepam tablets and all medicines out of the reach of children.
General Information about clonazepam tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use clonazepam tablets for a condition for which it was not prescribed. Do not give clonazepam tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about clonazepam tablets. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about clonazepam tablets that is written for health professionals.
What are the ingredients in clonazepam tablets?
Active Ingredient: clonazepam
Inactive Ingredients:
- Tablets
- 0.5 mg tablets contain anhydrous lactose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, starch (corn) and FD&C Yellow No. 6 Lake.
- 1 mg tablets contain anhydrous lactose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, starch (corn) and FD&C Blue No. 2 Lake.
- 2 mg tablets contain anhydrous lactose, lactose monohydrate, magnesium stearate, microcrystalline cellulose and starch (corn)
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured For:
Accord Healthcare, Inc.,
1009, Slater Road,
Suite 210-B,
Durham, NC 27703,
USA.
Manufactured By:
Intas Pharmaceuticals Limited,
Plot No. : 457, 458,
Village – Matoda,
Bavla Road, Ta.- Sanand,
Dist.- Ahmedabad – 382 210.
India.
10 7744 1 626035
Issued October, 2010.
| CLONAZEPAM
clonazepam tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| CLONAZEPAM
clonazepam tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - St Marys Medical Park Pharmacy (063050751) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| St Marys Medical Park Pharmacy | 063050751 | relabel(60760-300, 60760-273) , repack(60760-300, 60760-273) | |