HEAD AND SHOULDERS DRY SCALP- pyrithione zinc lotion/shampoo
Procter & Gamble Manufacturing Co
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
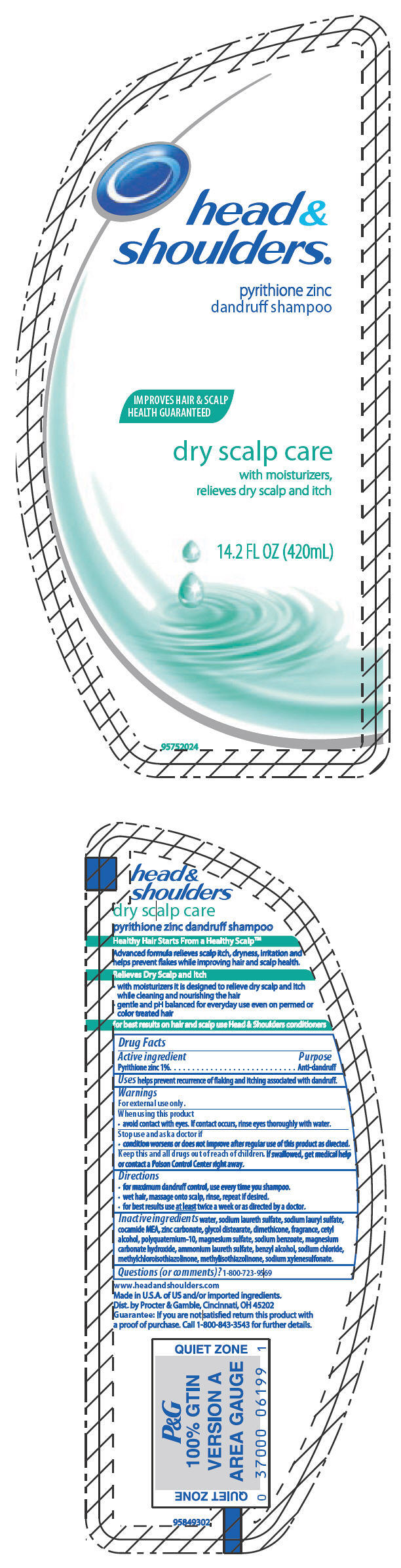
head & shoulders®
pyrithione zinc
dandruff shampoo
Warnings
For external use only.
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- for maximum dandruff control, use every time you shampoo.
- wet hair, massage onto scalp, rinse, repeat if desired.
- for best results use at least twice a week or as directed by a doctor.
Inactive ingredients
water, sodium laureth sulfate, sodium lauryl sulfate, cocamide MEA, zinc carbonate, glycol distearate, dimethicone, fragrance, cetyl alcohol, polyquaternium-10, magnesium sulfate, sodium benzoate, magnesium carbonate hydroxide, ammonium laureth sulfate, benzyl alcohol, sodium chloride, methylchloroisothiazolinone, methylisothiazolinone, sodium xylenesulfonate.
| HEAD AND SHOULDERS
DRY SCALP
pyrithione zinc lotion/shampoo |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Co (004238200) |
Revised: 1/2013
Document Id: 246ba94b-2f14-48ad-962c-5fb9fac1361c
Set id: c23dc070-87f4-480f-b04b-967e42462ddb
Version: 3
Effective Time: 20130115
Procter & Gamble Manufacturing Co