DILAUDID-HP
-
hydromorphone hydrochloride solution
DILAUDID-HP
-
hydromorphone hydrochloride powder
AbbVie Inc.
----------
WARNING: DILAUDID-HP ® (HIGH POTENCY) IS A HIGHLY CONCENTRATED SOLUTION OF HYDROMORPHONE, A POTENT SCHEDULE II CONTROLLED OPIOID AGONIST, INTENDED FOR USE IN OPIOID-TOLERANT PATIENTS. DO NOT CONFUSE DILAUDID-HP WITH STANDARD PARENTERAL FORMULATIONS OF DILAUDID OR OTHER OPIOIDS. OVERDOSE AND DEATH COULD RESULT.
SCHEDULE II OPIOID AGONISTS, INCLUDING MORPHINE, OXYMORPHONE, OXYCODONE, FENTANYL AND METHADONE, HAVE THE HIGHEST POTENTIAL FOR ABUSE AND RISK OF PRODUCING RESPIRATORY DEPRESSION. ALCOHOL, OTHER OPIOIDS AND CENTRAL NERVOUS SYSTEM DEPRESSANTS (SEDATIVE-HYPNOTICS) POTENTIATE THE RESPIRATORY DEPRESSANT EFFECTS OF HYDROMORPHONE, INCREASING THE RISK OF RESPIRATORY DEPRESSION THAT MIGHT RESULT IN DEATH.
DESCRIPTION
DILAUDID (hydromorphone hydrochloride), a hydrogenated ketone of morphine, is an opioid analgesic. HIGH POTENCY DILAUDID is available in AMBER ampules or single dose vials for intravenous (IV), subcutaneous (SC), or intramuscular (IM) administration. Each 1 mL of sterile solution contains 10 mg hydromorphone hydrochloride with 0.2% sodium citrate, and 0.2% citric acid solution.
It is also available as lyophilized DILAUDID for intravenous (IV), subcutaneous (SC), or intramuscular (IM) administration. Each single dose vial contains 250 mg sterile, lyophilized hydromorphone HCl to be reconstituted with 25 mL of Sterile Water for Injection USP to provide a solution containing 10 mg/mL.
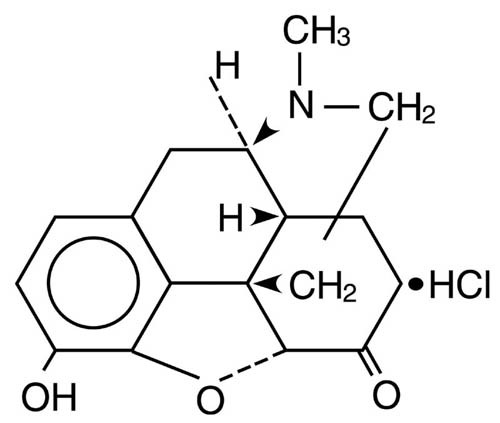
The chemical name of DILAUDID (hydromorphone hydrochloride) is 4,5α-epoxy-3-hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula is:
CLINICAL PHARMACOLOGY
Hydromorphone hydrochloride is a pure opioid agonist with the principal therapeutic activity of analgesia. A significant feature of the analgesia is that it can occur without loss of consciousness. Opioid analgesics also suppress the cough reflex and may cause respiratory depression, mood changes, mental clouding, euphoria, dysphoria, nausea, vomiting and electroencephalographic changes. Many of the effects described below are common to the class of mu-opioid analgesics, which includes morphine, oxycodone, hydrocodone, codeine, and fentanyl. In some instances, data may not exist to demonstrate that DILAUDID-HP possesses similar or different effects than those observed with other opioid analgesics. However, in the absence of data to the contrary, it is assumed that DILAUDID-HP would possess these effects.
Central Nervous System
The precise mode of analgesic action of opioid analgesics is unknown. However, specific CNS opiate receptors have been identified. Opioids are believed to express their pharmacological effects by combining with these receptors.
Hydromorphone depresses the cough reflex by direct effect on the cough center in the medulla.
Hydromorphone produces respiratory depression by direct effect on brain stem respiratory centers. The mechanism of respiratory depression also involves a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension.
Hydromorphone causes miosis. Pinpoint pupils are a common sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in the setting of DILAUDID overdose.
Gastrointestinal Tract and Other Smooth Muscle
Gastric, biliary and pancreatic secretions are decreased by opioids such as hydromorphone. Hydromorphone causes a reduction in motility associated with an increase in tone in the gastric antrum and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, and tone may be increased to the point of spasm. The end result is constipation. Hydromorphone can cause a marked increase in biliary tract pressure as a result of spasm of the sphincter of Oddi.
Cardiovascular System
Hydromorphone may produce hypotension as a result of either peripheral vasodilation, release of histamine, or both. Other manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, and red eyes.
Effects on the myocardium after intravenous administration of opioids are not significant in normal persons, vary with different opioid analgesic agents and vary with the hemodynamic state of the patient, state of hydration and sympathetic drive.
Pharmacokinetics and Metabolism
Distribution
At therapeutic plasma levels, hydromorphone is approximately 8-19% bound to plasma proteins. After an intravenous bolus dose, the steady state of volume of distribution [mean (%cv)] is 302.9 (32%) liters.
Metabolism
Hydromorphone is extensively metabolized via glucuronidation in the liver, with greater than 95% of the dose metabolized to hydromorphone-3-glucuronide along with minor amounts of 6-hydroxy reduction metabolites.
Elimination
Only a small amount of the hydromorphone dose is excreted unchanged in the urine. Most of the dose is excreted as hydromorphone-3-glucuronide along with minor amounts of 6-hydroxy reduction metabolites. The systemic clearance is approximately 1.96 (20%) liters/minute. The terminal elimination half-life of hydromorphone after an intravenous dose is about 2.3 hours.
Special Populations
Hepatic Impairment
After oral administration of hydromorphone at a single 4 mg dose (2 mg Dilaudud IR Tablets), mean exposure to hydromorphone (Cmax and AUC∞) is increased 4 fold in patients with moderate (Child-Pugh Group B) hepatic impairment compared with subjects with normal hepatic function. Due to increased exposure of hydromorphone, patients with moderate hepatic impairment should be started at a lower dose and closely monitored during dose titration. Pharmacokinetics of hydromorphone in severe hepatic impairment patients has not been studied. Further increase in Cmax and AUC of hydromorphone in this group is expected. As such, starting dose should be even more conservative. Use of oral liquid is recommended to adjust the dose (see DOSAGE AND ADMINISTRATION).
Renal Impairment
After oral administration of hydromorphone at a single 4 mg dose (2 mg Dilaudid IR Tablets), mean exposure to hydromorphone (Cmax and AUC0-48) is increased in patients with impaired renal function by 2-fold, in moderate (CLcr = 40 - 60 mL/min) and 3-fold in severe (CLcr< 30 mL/min) renal impairment compared with normal subjects (CLcr > 80 mL/min). In addition, in patients with severe renal impairment hydromorphone appeared to be more slowly eliminated with longer terminal elimination half-life (40 hr) compared to patients with normal renal function (15 hr). Patients with moderate renal impairment should be started on a lower dose. Starting doses for patients with severe renal impairment should be even lower. Patients with renal impairment should be closely monitored during dose titration. Use of oral liquid is recommended to adjust the dose (see DOSAGE AND ADMINISTRATION).
INDICATIONS AND USAGE
DILAUDID-HP is indicated for the relief of moderate-to-severe pain in opioid-tolerant patients who require larger than usual doses of opioids to provide adequate pain relief. Because DILAUDID-HP contains 10 mg of hydromorphone hydrochloride per mL, a smaller injection volume can be used than with other parenteral opioid formulations. Discomfort associated with the intramuscular or subcutaneous injection of an unusually large volume of solution can therefore be avoided.
CONTRAINDICATIONS
DILAUDID-HP is contraindicated in: patients who are not already receiving large amounts of parenteral opioids, patients with known hypersensitivity to hydromorphone, patients with respiratory depression in the absence of resuscitative equipment, and in patients with status asthmaticus. DILAUDID-HP is also contraindicated for use in obstetrical analgesia.
WARNINGS
Respiratory Depression
Respiratory depression is the chief hazard of DILAUDID-HP. Respiratory depression occurs most frequently in overdose situations, in the elderly, in the debilitated, and in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation.
DILAUDID-HP should be used with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale, patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or preexisting respiratory depression. In such patients even usual therapeutic doses of opioid analgesics may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea.
DILAUDID-HP contains hydromorphone, which is a potent Schedule II, controlled opioid agonist. Schedule II opioid agonists, including morphine, oxycodone, oxymorphone, fentanyl and methadone, have the highest potential for abuse and risk of fatal respiratory depression. Alcohol, other opioids and central nervous system depressants (sedative-hypnotics) potentiate the respiratory depressant effects of hydromorphone, increasing the risk of respiratory depression that might result in death.
Misuse, Abuse, and Diversion of Opioids
Hydromorphone is an opioid agonist of the morphine-type. Such drugs are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
DILAUDID-HP can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing DILAUDID in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion. Prescribers should monitor all patients receiving opioids for signs of abuse, misuse, and addiction. Furthermore, patients should be assessed for their potential for opioid abuse prior to being prescribed opioid therapy. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse) or mental illness (e.g., depression). Opioids may still be appropriate for use in these patients, however, they will require intensive monitoring for signs of abuse.
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain.
Healthcare professionals should contact their State Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Interactions with Alcohol and Drugs of Abuse
Hydromorphone may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression.
Neonatal Withdrawal Syndrome
Infants born to mothers physically dependent on DILAUDID-HP will also be physically dependent and may exhibit respiratory difficulties and withdrawal symptoms. (see DRUG ABUSE AND DEPENDENCE).
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of DILAUDID-HP with carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions, or preexisting increase in intracranial pressure. Opioid analgesics including DILAUDID-HP may produce effects on pupillary response and consciousness which can obscure the clinical course and neurologic signs of further increase in pressure in patients with head injuries.
Hypotensive Effect
Opioid analgesics, including DILAUDID-HP, may cause severe hypotension in an individual whose ability to maintain his blood pressure has already been compromised by a depleted blood volume, or a concurrent administration of drugs such as phenothiazines or general anesthetics (see PRECAUTIONS - Drug Interactions). DILAUDID-HP may produce orthostatic hypotension in ambulatory patients.
DILAUDID-HP should be administered with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
Sulfites
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
PRECAUTIONS
General
Because of its high concentration, the delivery of precise doses of DILAUDID-HP may be difficult if low doses of hydromorphone are required. Therefore, DILAUDID-HP should be used only if the amount of hydromorphone required can be delivered accurately with this formulation.
Special Risk Patients
DILAUDID-HP should be given with caution and the initial dose should be reduced in the elderly or debilitated and those with severe impairment of hepatic, pulmonary or renal function; myxedema or hypothyroidism; adrenocortical insufficiency (e.g., Addison's Disease); CNS depression or coma; toxic psychoses; prostatic hypertrophy or urethral stricture; gall bladder disease; acute alcoholism; delirium tremens; or kyphoscoliosis, or following gastrointestinal surgery.
In the case of DILAUDID-HP, however, the patient is presumed to be receiving an opioid to which he or she exhibits tolerance and the initial dose of DILAUDID-HP selected should be estimated based on the relative potency of hydromorphone and the opioid previously used by the patient. (see DOSAGE AND ADMINISTRATION).
The administration of opioid analgesics including DILAUDID-HP may obscure the diagnosis or clinical course in patients with acute abdominal conditions and may aggravate preexisting convulsions in patients with convulsive disorders.
Reports of mild to severe seizures and myoclonus have been reported in severely compromised patients, administered high doses of parenteral hydromorphone, for cancer and severe pain. Opioid administration at very high doses is associated with seizures and myoclonus in a variety of diseases where pain control is the primary focus.
Use in Drug and Alcohol Dependent Patients
DILAUDID-HP should be used with caution in patients with alcoholism and other drug dependencies due to the increased frequency of opioid tolerance, dependence, and the risk of addiction observed in these patient populations. Abuse of DILAUDID-HP in combination with other CNS depressant drugs can result in serious risk to the patient.
Hydromorphone is an opioid with no approved use in the management of addictive disorders.
Use in Ambulatory Patients
DILAUDID-HP may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g. driving, operating machinery). Patients should be cautioned accordingly. DILAUDID may produce orthostatic hypotension in ambulatory patients.
Use in Biliary Tract Disease
Opioid analgesics, including DILAUDID-HP, should also be used with caution in patients about to undergo surgery of the biliary tract since it may cause spasm of the sphincter of Oddi.
Tolerance and Physical Dependence
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist. Physical dependence and tolerance are not unusual during chronic opioid therapy.
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, mydriasis. Other symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
In general, opioids used regularly should not be abruptly discontinued.
Drug Interactions
Drug Interactions with other CNS Depressants
The concomitant use of other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers and alcohol may produce additive depressant effects. Respiratory depression, hypotension and profound sedation or coma may occur. When such combined therapy is contemplated, the dose of one or both agents should be reduced. Opioid analgesics, including DILAUDID-HP, may enhance the action of neuromuscular blocking agents and produce an increased degree of respiratory depression.
Interactions with Mixed Agonist/Antagonist Opioid Analgesics
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol, and buprenorphine) should be administered with caution to a patient who has received or is receiving a course of therapy with a pure opioid agonist analgesic such as hydromorphone. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of hydromorphone and/or may precipitate withdrawal symptoms in these patients.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted in animals.
Hydromorphone was not mutagenic in the in vitro Ames reverse mutation assay, or the human lymphocytes chromosome aberration assay. Hydromorphone was not clastogenic in the in vivo mouse micronucleus assay.
No effects on fertility, reproductive performance, or reproductive organ morphology were observed in male or female rats given oral doses up to 7 mg/kg/day which is equivalent to and 3-fold higher than the human dose of DILAUDID-HP when substituted for ORAL LIQUID or 8 mg TABLET, respectively, on a body surface area basis.
PREGNANCY
PREGNANCY CATEGORY C
No effects on teratogenicity or embryotoxicity were observed in female rats given oral doses up to 7 mg/kg/day which is equivalent to and 3-fold higher than the human dose of DILAUDID-HP, on a body surface area basis. Hydromorphone produced skull malformations (exencephaly and cranioschisis) in Syrian hamsters given oral doses up to 20 mg/kg during the peak of organogenesis (gestation days 8-9). The skull malformations were observed at doses approximately 2-fold and 7-fold higher than the human dose of DILAUDID-HP when substituted for ORAL LIQUID or 8 mg TABLET, respectively, on a body surface area basis. There are no adequate and well-controlled studies of DILAUDID in pregnant women.
Hydromorphone crosses the placenta, resulting in fetal exposures. DILAUDID-HP should be used in pregnant women only if the potential benefit justifies the potential risk to the fetus (see Labor and Delivery and DRUG ABUSE AND DEPENDENCE).
Nonteratogenic Effects
Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal. Approaches to the treatment of this syndrome have included supportive care and, when indicated, drugs such as paregoric or phenobarbital.
Nursing Mothers
Low levels of opioid analgesics have been detected in human milk. As a general rule, nursing should not be undertaken while a patient is receiving DILAUDID-HP since it, and other drugs in this class, may be excreted in the milk.
Geriatric Use
Clinical studies of DILAUDID did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. (see PRECAUTIONS).
ADVERSE REACTIONS
The major hazards of DILAUDID-HP include respiratory depression and apnea. To a lesser degree, circulatory depression, respiratory arrest, shock and cardiac arrest have occurred.
The most frequently observed adverse effects are lightheadedness, dizziness, sedation, nausea, vomiting, sweating, flushing, dysphoria, euphoria, dry mouth, and pruritus. These effects seem to be more prominent in ambulatory patients and in those not experiencing severe pain.
Less Frequently Observed Adverse Reactions
General and CNS
Weakness, headache, agitation, tremor, uncoordinated muscle movements, alterations of mood (nervousness, apprehension, depression, floating feelings, dreams), muscle rigidity, paresthesia, muscle tremor, blurred vision, nystagmus, diplopia and miosis, transient hallucinations and disorientation, visual disturbances, insomnia, increased intracranial pressure
Cardiovascular
Flushing of the face, chills, tachycardia, bradycardia, palpitation, faintness, syncope, hypotension, hypertension
Gastrointestinal
Constipation, biliary tract spasm, ileus, anorexia, diarrhea, cramps, taste alterations
Dermatologic
Urticaria, other skin rashes, wheal and flare over the vein with intravenous injection, diaphoresis
Other
In clinical trials, neither local tissue irritation nor induration was observed at the site of subcutaneous injection of DILAUDID-HP; pain at the injection site was rarely observed. However, local irritation and induration have been seen following parenteral injection of other opioid drug products.
OVERDOSAGE
Serious overdosage with DILAUDID-HP is characterizedby respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and sometimes bradycardia and hypotension. In serious overdosage, particularly following intravenous injection, apnea, circulatory collapse, cardiac arrest and death may occur.
In the treatment of overdosage, primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen, vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
The opioid antagonist, naloxone, is a specific antidote against respiratory depression which may result from overdosage, or unusual sensitivity to DILAUDID-HP. Naloxone should not be administered in the absence of clinically significant respiratory or circulatory depression. Naloxone should be administered cautiously to persons who are known, or suspected to be physically dependent on DILAUDID-HP. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute withdrawal syndrome.
Since the duration of action of DILAUDID-HP may exceed that of the antagonist, the patient should be kept under continued surveillance; repeated doses of the antagonist may be required to maintain adequate respiration. Apply other supportive measures when indicated.
DOSAGE AND ADMINISTRATION
Parenteral
DILAUDID-HP SHOULD BE GIVEN ONLY TO PATIENTS WHO ARE ALREADY RECEIVING LARGE DOSES OF OPIOIDS. DILAUDID-HP is indicated for relief of moderate-to-severe pain in opioid-tolerant patients. Thus, these patients will already have been treated with other opioid analgesics. If the patient is being changed from regular DILAUDID to DILAUDID-HP, similar doses should be used, depending on the patient's clinical response to the drug. If DILAUDID-HP is substituted for a different opioid analgesic, the following equivalency table should be used as a guide to determine the appropriate dose of DILAUDID-HP (hydromorphone hydrochloride). Patients with hepatic and renal impairment should be started on a lower starting dose (See CLINICAL PHARMACOLOGY - Pharmacokinetics and Metabolism). The dosage of DILAUDID-HP should be individualized for any given patient, since adverse events can occur at doses that may not provide complete freedom from pain.
Safe and effective administration of opioid analgesics to patients with acute or chronic pain depends upon a comprehensive assessment of the patient. The nature of the pain (severity, frequency, etiology, and pathophysiology) as well as the concurrent medical status of the patient will affect selection of the starting dosage.
In open clinical trials with DILAUDID-HP in patients with terminal cancer, doses ranged from 1-14 mg subcutaneously or intramuscularly; one patient received 30 mg subcutaneously on two occasions. In these trials, both subcutaneous and intramuscular injections of DILAUDID-HP were well-tolerated, with minimal pain and/or burning at the injection site. Mild erythema was rarely noted after intramuscular injection. There was no induration after either intramuscular or subcutaneous administration of DILAUDID-HP. Subcutaneous injections of DILAUDID-HP were particularly well accepted when administered with a short, 30 gauge needle.
Experience with administration of DILAUDID-HP by the intravenous route is limited. Should intravenous administration be necessary, the injection should be given slowly, over at least 2 to 3 minutes. The intravenous route is usually painless.
A gradual increase in dose may be required if analgesia is inadequate, tolerance occurs, or if pain severity increases. The first sign of tolerance is usually a reduced duration of effect.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. A slight yellowish discoloration may develop in DILAUDID-HP ampules. No loss of potency has been demonstrated. DILAUDID injection is physically compatible and chemically stable for at least 24 hours at 25°C protected from light in most common large volume parenteral solutions.
500 mg/50 mL Vial
To use this single dose presentation, do not penetrate the stopper with a syringe. Instead, remove both the aluminum flipseal and rubber stopper in a suitable work area such as under a laminar flow hood (or equivalent clean air compounding area). The contents may then be withdrawn for preparation of a single, large volume parenteral solution. Any unused portion should be discarded in an appropriate manner.
CAUTION: The packaging (vial stopper) of this product contains rubber latex which may cause allergic reactions.
DRUG ABUSE AND DEPENDENCE
DILAUDID-HP contains hydromorphone, a Schedule II controlled opioid agonist. Schedule II opioid substances which include morphine, oxycodone, oxymorphone, fentanyl, and methadone have the highest potential for abuse and risk of fatal overdose. Hydromorphone can be abused and is subject to criminal diversion.
Opioid analgesics may cause psychological and physical dependence. Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug. Physical dependence usually does not occur to a clinically significant degree until after several weeks of continued opioid usage, but it may occur after as little as a week of opioid use. Physical dependence and tolerance are separate and distinct from abuse and addiction.
Addiction is a chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
“Drug seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with, forging or counterfeiting prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s).“Doctor shopping” to obtain additional prescriptions is common among drug abusers, people suffering from untreated addiction and criminals seeking drugs to sell.
Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Since DILAUDID may be diverted for non-medical use, careful record keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
DILAUDID-HP is intended for parenteral use only under the direct supervision of an appropriately licensed health care provider. Misuse or abuse of DILAUDID-HP poses a risk of overdose and death. This risk is increased with concurrent abuse of alcohol and other substances. Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
SAFETY AND HANDLING INSTRUCTIONS
DILAUDID-HP poses little risk of direct exposure to health care personnel and should be handled and disposed of prudently in accordance with hospital or institutional policy. Patients and their families should be instructed to flush any DILAUDID-HP that is no longer needed.
Access to abusable drugs such as DILAUDID-HP presents an occupational hazard for addiction in the health care industry. Routine procedures for handling controlled substances developed to protect the public may not be adequate to protect health care workers. Implementation of more effective accounting procedures and measures to restrict access to drugs of this class (appropriate to the practice setting) may minimize the risk of self-administration by health care providers.
HOW SUPPLIED
DILAUDID-HP amber ampules and single dose vials contain 10 mg hydromorphone hydrochloride per mL with 0.2% sodium citrate and 0.2% citric acid solution. No added preservative.
NOTE: DILAUDID-HP ampules are amber in color.
The lyophilized DILAUDID-HP Single Dose Vial contains 250 mg of sterile, lyophilized hydromorphone HCl.
| DILAUDID-HP
hydromorphone hydrochloride solution |
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019034 | 01/11/1984 | 04/30/2008 |
| DILAUDID-HP
hydromorphone hydrochloride powder |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019034 | 01/11/1984 | 04/30/2008 |
| Labeler - AbbVie Inc. (078458370) |
Revised: 01/2013 AbbVie Inc.