flurbiprofen (Flurbiprofen) tablet, film coated
[TEVA PHARMACEITICALS USA]
DESCRIPTION
Flurbiprofen is a nonsteroidal anti-inflammatory agent. Flurbiprofen is a phenylalkanoic acid derivative designated chemically as (±)-2-(2-fluoro-4-biphenylyl)propionic acid. Flurbiprofen is a white or slightly yellow crystalline powder. It is slightly soluble in water at pH 7.0 and readily soluble in most polar solvents. Its structural formula is:
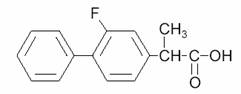
C15H13FO2, M.W. 244.26
Each tablet, for oral administration, contains 100 mg flurbiprofen. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, titanium dioxide and FD&C Blue #1 aluminum lake.
CLINICAL PHARMACOLOGY
Flurbiprofen is a nonsteroidal anti-inflammatory agent which has shown anti-inflammatory, analgesic, and antipyretic properties in pharmacologic studies. As with other such drugs, its mode of action is not known. However, it is a potent prostaglandin synthesis inhibitor, and this property may be involved in its anti-inflammatory effect.
Flurbiprofen is well absorbed after oral administration, reaching peak blood levels in approximately 1.5 hours (range 0.5 to 4 hours). Administration with food alters the rate of absorption but does not affect the extent of drug availability. The elimination half-life is approximately 6 hours with 90% of the half-life values from 3 to 9 hours. Individual half-life values ranged from 2.8 to 12 hours. There is no evidence of drug accumulation and flurbiprofen does not induce enzymes that alter its metabolism. Excretion of flurbiprofen is 88% to 98% complete 24 hours after the last dose.
Flurbiprofen is extensively metabolized and excreted primarily in the urine, about 20% as free and conjugated drug and about 50% as hydroxylated metabolites. About 90% of the flurbiprofen in urine is present as conjugates. The major metabolite, 4’-hydroxy-flurbiprofen, has been detected in human plasma, but in animal models of inflammation this metabolite showed little anti-inflammatory activity. Flurbiprofen is more than 99% bound to human serum proteins.
In a reported study the average maximum serum concentration of flurbiprofen, following a 100 mg oral dose of flurbiprofen tablets in normal volunteers (n=184), was 15.2 µg/mL, with 90% of the values between 10 and 22 µg/mL. In geriatric subjects (n=7) between the ages of 58 and 77 years, 100 mg flurbiprofen resulted in an average peak drug level of 18.0 µg/mL and an average elimination half-life of 6.5 hours (range 3-10 hours). In geriatric rheumatoid arthritis patients (n=13) between the ages of 65 and 83 years receiving 100 mg flurbiprofen, the average maximum blood level was 12.7 µg/mL and the average elimination half-life was 5.6 hours (range 4-10 hours).
In a study assessing flurbiprofen pharmacokinetics in end stage renal disease (ESRD), mean urinary recovery of a 100 mg dose was 73% in 48 hours for 9 normal subjects and 17% in 96 hours for 8 ESRD patients undergoing continuous ambulatory peritoneal dialysis. Plasma concentrations of flurbiprofen were about 40% lower in the ESRD patients; the elimination half-life of flurbiprofen was unchanged. Elimination of the 4’-hydroxy-flurbiprofen metabolite was markedly reduced in the ESRD patients. The pharmacokinetics of flurbiprofen in patients with decreased renal function but not ESRD have not been determined.
The pharmacokinetics of flurbiprofen in patients with hepatic disease have not been determined.
The efficacy of flurbiprofen has been demonstrated in patients with rheumatoid arthritis and osteoarthritis. Using standard assessments of therapeutic response, flurbiprofen (200-300 mg/day) demonstrated effectiveness comparable to aspirin (2000-4000 mg/day), ibuprofen (2400-3200 mg/day), and indomethacin (75-150 mg/day).
In patients with rheumatoid arthritis, flurbiprofen may be used in combination with gold salts or corticosteroids.
INDICATIONS AND USAGE
Flurbiprofen tablets are indicated for the acute or long-term treatment of the signs and symptoms of rheumatoid arthritis and osteoarthritis.
CONTRAINDICATIONS
Flurbiprofen tablets are contraindicated in patients who have previously demonstrated hypersensitivity to the product. Flurbiprofen should not be given to patients in whom flurbiprofen, aspirin, or other nonsteroidal anti-inflammatory drugs induce asthma, urticaria, or other allergic-type reactions. Fatal asthmatic reactions have been reported in such patients receiving this type of drug.
WARNINGS
Risk of Gastrointestinal (GI) Ulcerations, Bleeding and Perforation with Nonsteroidal Anti-inflammatory Therapy
Serious gastrointestinal toxicity, such as bleeding, ulceration, and perforation, can occur at any time, with or without warning symptoms, in patients treated chronically with nonsteroidal anti-inflammatory drugs. Although minor upper GI problems, such as dyspepsia, are common, usually developing early in therapy, physicians should remain alert for ulceration and bleeding in patients treated chronically with nonsteroidal anti-inflammatory drugs, even in the absence of previous GI tract symptoms. In patients observed in clinical trials of such agents for several months to two years, symptomatic upper GI ulcers, gross bleeding, or perforation appear to occur in approximately 1% of patients treated for 3-6 months, and in about 2-4% of patients treated for one year. Physicians should inform patients about the signs and/or symptoms of serious GI toxicity and what steps to take if they occur.
Studies to date have not identified any subset of patients not at risk of developing peptic ulceration and bleeding. Except for a prior history of serious GI events and other risk factors known to be associated with peptic ulcer disease, such as alcoholism, smoking, etc., no risk factors (e.g., age, sex) have been associated with increased risk. Elderly or debilitated patients seem to tolerate ulceration or bleeding less well than other individuals and most spontaneous reports of fatal GI events are in this population. Studies to date are inconclusive concerning the relative risk of various nonsteroidal anti-inflammatory agents in causing such reactions. High doses of any such agent probably carry a greater risk of these reactions, although controlled clinical trials showing this do not exist in most cases. In considering the use of relatively large doses (within the recommended dosage range), sufficient benefit should be anticipated to offset the potential increased risk of GI toxicity.
Because serious GI tract ulceration and bleeding can occur without warning symptoms, physicians should follow chronically treated patients for the signs and symptoms of ulceration and bleeding and should inform the patients of the importance of this follow-up.
PRECAUTIONS
General Precautions
Impaired Renal or Hepatic Function
As with other nonsteroidal anti-inflammatory drugs, flurbiprofen should be used with caution in patients with impaired renal or hepatic function, or a history of kidney or liver disease. Studies to assess the pharmacokinetics of flurbiprofen in patients with decreased liver function have not been done.
Renal Effects
Toxicology studies in rats have shown renal papillary necrosis at dosage levels equivalent on a mg/kg basis to those used clinically in humans. Similar findings were seen in monkeys given high doses (50-100 mg/kg, or approximately 20-40 times the human therapeutic dose) for 90 days.
In clinical studies, kidney function tests were done at least monthly in patients taking flurbiprofen. In these studies, renal effects of flurbiprofen were similar to those seen with other nonsteroidal anti-inflammatory drugs.
A second form of renal toxicity has been seen in patients with prerenal conditions leading to a reduction in renal blood flow or blood volume, where the renal prostaglandins have a supportive role in the maintenance of renal perfusion. In these patients administration of a nonsteroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics, and the elderly. Discontinuation of nonsteroidal anti-inflammatory drug therapy is typically followed by recovery to the pretreatment state. Those patients at high risk who chronically take flurbiprofen should have renal function monitored if they have signs or symptoms that may be consistent with mild azotemia, such as malaise, fatigue, loss of appetite, etc. Occasional patients may develop some elevation of serum creatinine and BUN levels without signs or symptoms.
The elimination half-life of flurbiprofen was unchanged in patients with end stage renal disease (ESRD). Flurbiprofen metabolites are primarily eliminated by the kidneys and elimination of 4’-hydroxy-flurbiprofen was markedly reduced in ESRD patients. Therefore, patients with significantly impaired renal function may require a reduction of dosage to avoid accumulation of flurbiprofen metabolites and should be monitored. (See also the CLINICAL PHARMACOLOGY section.)
Liver Tests
As with other nonsteroidal anti-inflammatory drugs, borderline elevations of one or more liver tests may occur in up to 15% of patients. These abnormalities may progress, may remain essentially unchanged, or may disappear with continued therapy. The ALT (SGPT) test is probably the most sensitive indicator of liver injury. Meaningful (3 times the upper limit of normal) elevations of ALT or AST (SGOT) have been reported in controlled clinical trials in less than 1% of patients. A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with flurbiprofen.
Anemia
Anemia is commonly observed in rheumatoid arthritis and is sometimes aggravated by nonsteroidal anti-inflammatory drugs, which may produce fluid retention or minor gastrointestinal blood loss in some patients. Therefore, patients who have initial hemoglobin values of 10 g/dL or less, and who are to receive long-term therapy, should have hemoglobin values determined periodically.
Fluid Retention and Edema
Fluid retention and edema have been reported; therefore, flurbiprofen should be used with caution in patients with cardiac decompensation, hypertension, or similar conditions.
Vision Changes
Blurred and/or diminished vision has been reported with the use of flurbiprofen and other nonsteroidal anti-inflammatory drugs. Patients experiencing eye complaints should have ophthalmologic examinations.
Effect on Platelets and Coagulation
Flurbiprofen inhibits collagen-induced platelet aggregation. Prolongation of bleeding time by flurbiprofen has been demonstrated in humans after single and multiple oral doses. Patients who may be adversely affected by prolonged bleeding time should be carefully observed when flurbiprofen is administered.
Information for Patients
Flurbiprofen, like other drugs of its class, is not free of side effects. The side effects of these drugs can cause discomfort and, rarely, there are more serious side effects, such as gastrointestinal bleeding, which may result in hospitalization and even fatal outcomes. Nonsteroidal anti-inflammatory drugs are often essential agents in the management of arthritis, but they also may be commonly employed for conditions which are less serious. Physicians may wish to discuss with their patients the potential risks (see WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS sections) and likely benefits of nonsteroidal anti-inflammatory drug treatment, particularly when the drugs are used for less serious conditions where treatment without such agents may represent an acceptable alternative to both the patient and the physician.
Drug Interactions
Antacids
Administration of flurbiprofen to volunteers under fasting conditions, or with antacid suspension, yielded similar serum flurbiprofen-time profiles in young subjects (n=12). In geriatric subjects (n=7) there was a reduction in the rate but not the extent of flurbiprofen absorption.
Anticoagulants
Flurbiprofen, like other nonsteroidal anti-inflammatory drugs, has been shown to affect bleeding parameters in patients receiving anticoagulants, and serious clinical bleeding has been reported. The physician should be cautious when administering flurbiprofen to patients taking anticoagulants.
Aspirin
Concurrent administration of aspirin and flurbiprofen resulted in 50% lower serum flurbiprofen concentrations. This effect of aspirin (which also lowers serum concentrations of other nonsteroidal anti-inflammatory drugs given with it) has been demonstrated in patients with rheumatoid arthritis (n=15) as well as normal volunteers (n=16). Concurrent use of flurbiprofen and aspirin is therefore not recommended.
Beta-adrenergic Blocking Agents
The effect of flurbiprofen on blood pressure response to propranolol and atenolol was evaluated in men with mild uncomplicated hypertension (n=10). Flurbiprofen pretreatment attenuated the hypotensive effect of a single dose of propranolol but not atenolol. Flurbiprofen did not appear to affect the beta-blocker-mediated reduction in heart rate. Flurbiprofen did not affect the pharmacokinetic profile of either drug, and the mechanism underlying the interference with propranolol’s hypotensive effect is unknown. Patients taking both flurbiprofen and a beta-blocker should be monitored to ensure that a satisfactory hypotensive effect is achieved.
Cimetidine, Ranitidine
In normal volunteers (n=9), pretreatment with cimetidine or ranitidine did not affect flurbiprofen pharmacokinetics, except that a small (13%) but statistically significant increase in the area under the serum concentration curve of flurbiprofen resulted with cimetidine.
Digoxin
Studies of concomitant administration of flurbiprofen and digoxin to healthy men (n=14) did not show a change in the steady state serum levels of either drug.
Diuretics
Studies in normal volunteers have shown that flurbiprofen, like other nonsteroidal anti-inflammatory drugs, can interfere with the effects of furosemide. Although results have varied from study to study, effects have been shown on furosemide-stimulated diuresis, natriuresis, and kaliuresis. Other nonsteroidal anti-inflammatory drugs that inhibit prostaglandin synthesis have been shown to interfere with thiazide diuretics in some studies, and with potassium-sparing diuretics. Patients receiving flurbiprofen and furosemide or other diuretics should be observed closely to determine if the desired effect is obtained.
Oral Hypoglycemic Agents
In one study, flurbiprofen was given to adult diabetics who were already receiving glyburide (n=4), metformin (n=2), chlorpropamide with phenformin (n=3), or glyburide with phenformin (n=6). Although there was a slight reduction in blood sugar concentrations during concomitant administration of flurbiprofen and hypoglycemic agents, there were no signs or symptoms of hypoglycemia.
Carcinogenesis, Mutagenesis, Impairment of Fertility
An 80-week study in mice at doses of 2, 5, and 12 mg/kg/day and a 2-year study in rats at doses of 0.5, 2, and 4 mg/kg/day did not show evidence of carcinogenicity at maximum tolerated doses of flurbiprofen.
Flurbiprofen did not impair the fertility of male or female rats treated orally at 2.25 mg/kg/day for 65 days and 16 days, respectively, before mating.
Pregnancy
Teratogenic Effects
Pregnancy Category B
In teratology studies flurbiprofen, given to mice in doses up to 12 mg/kg/day, to rats in doses up to 25 mg/kg/day, and to rabbits in doses up to 7.5 mg/kg/day, showed no teratogenic effects. Because there are no adequate and well-controlled studies in pregnant women, and animal teratology studies do not always predict human response, flurbiprofen is not recommended for use in pregnancy.
Labor and Delivery
Flurbiprofen’s effects on labor and delivery in women are not known. As with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia and delayed parturition occurred in rats treated throughout pregnancy. Because of the known effects of prostaglandin-inhibiting drugs on the fetal cardiovascular system (closure of the ductus arteriosus), use of flurbiprofen during late pregnancy is not recommended.
Nursing Mothers
Concentrations of flurbiprofen in breast milk and plasma of nursing mothers suggested that a nursing infant could receive approximately 0.10 mg flurbiprofen per day in the established milk of a woman taking 200 mg/day. Because of possible adverse effects of prostaglandin-inhibiting drugs on neonates, flurbiprofen is not recommended for use in nursing mothers.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS
Adverse reaction information was derived from patients who received flurbiprofen in blinded-controlled and open-label clinical trials, and from worldwide marketing experience and from publications. In the description below, rates of the more common events (greater than 1%) and many of the less common events (less than 1%) represent clinical study results. For rarer events that were derived principally from worldwide marketing experience and the literature (printed in italics), accurate rate estimates are generally impossible.
Of the 4123 patients in premarketing studies, 2954 were treated for at least 1 month, 1448 for at least 3 months, 948 for at least 6 months, 356 for at least 1 year, and 100 for at least 2 years. Of the 4123 patients, 9.4% dropped out of the studies because of an adverse drug reaction, principally involving the gastrointestinal tract (5.8%), central nervous system and special senses (1.4%), skin (0.6%) and genitourinary tract (0.5%).
Incidence Greater Than 1%
An asterisk after a reaction identifies reactions which occurred in 3-9% of patients treated with flurbiprofen. Reactions occurring in 1-3% of the patients are unmarked.
Gastrointestinal: Dyspepsia*, diarrhea*, abdominal pain*, nausea*, constipation, GI bleeding, flatulence, elevated liver enzymes, and vomiting.
Central Nervous System: Headache*, nervousness, and other manifestations of CNS “stimulation” (e.g., anxiety, insomnia, reflexes increased, and tremor), and symptoms associated with CNS “inhibition” (e.g., amnesia, asthenia, somnolence, malaise, and depression).
Respiratory: Rhinitis.
Dermatological: Rash.
Special Senses: Dizziness, tinnitus, and changes in vision.
Genitourinary: Signs and symptoms suggesting urinary tract infection*.
Body as a Whole: Edema*.
Metabolic/Nutritional: Body weight changes.
Incidence Less Than 1% (Causal Relationship Probable)
The reactions listed in this category occurred in <1% of patients in the clinical trials or were reported during postmarketing experience from other countries. Adverse reactions reported only in worldwide postmarketing experience or the literature (which presumably indicates that they are rarer) are italicized.
Gastrointestinal: Peptic ulcer disease (see also WARNINGS, Risk of Gastrointestinal (GI) Ulcerations, Bleeding and Perforation with Nonsteroidal Anti-inflammatory Therapy), gastritis, bloody diarrhea, stomatitis, esophageal disease, hematemesis, and hepatitis; cholestatic and non-cholestatic jaundice.
Central Nervous System: Ataxia, cerebrovascular ischemia, confusion, paresthesia, and twitching.
Hematologic: Decrease in hemoglobin and hematocrit, iron deficiency anemia, hemolytic anemia and aplastic anemia; leukopenia; eosinophilia; ecchymosis and thrombocytopenia. (See also PRECAUTIONS, Effect on Platelets and Coagulation.)
Respiratory: Asthma and epistaxis.
Dermatological: Angioedema, urticaria, eczema, and pruritus; photosensitivity, toxic epidermal necrolysis, and exfoliative dermatitis.
Special Senses: Conjunctivitis and parosmia.
Genitourinary: Hematuria and renal failure; interstitial nephritis.
Body as a Whole: Chills and fever; anaphylactic reaction.
Metabolic/Nutritional: Hyperuricemia.
Cardiovascular: Heart failure, hypertension, vascular diseases and vasodilation.
Incidence Less Than 1% (Causal Relationship Unknown)
The following reactions have been reported in patients taking flurbiprofen under circumstances that do not permit a clear attribution of the reaction to flurbiprofen. These reactions are being included as alerting information for physicians. Adverse reactions reported only in worldwide postmarketing experience or the literature (which presumably indicates that they are rarer) are italicized.
Gastrointestinal: Periodontal abscess, appetite changes, cholecystitis, and dry mouth.
Central Nervous System: Convulsion, meningitis, hypertonia, cerebrovascular accident, emotional lability, and subarachnoid hemorrhage.
Hematologic: Lymphadenopathy.
Respiratory: Bronchitis, laryngitis, dyspnea, pulmonary embolism, pulmonary infarct, and hyperventilation.
Dermatological: Alopecia, nail disorder, herpes simplex, zoster, dry skin, and sweating.
Special Senses: Ear disease, corneal opacity, glaucoma, retrobulbar neuritis, changes in taste, and transient hearing loss; retinal hemorrhage.
Genitourinary: Menstrual disturbances, vaginal and uterine hemorrhage, vulvovaginitis, and
prostate disease.
Metabolic/Nutritional: Hyperkalemia.
Cardiovascular: Arrhythmias, angina pectoris, and myocardial infarction.
Musculoskeletal: Myasthenia.
DRUG ABUSE AND DEPENDENCE
No drug abuse or drug dependence has been observed with flurbiprofen.
OVERDOSAGE
Information on overdosage is available for 13 children and 12 adults. Nine of the 13 children were less than 6 years old. Drowsiness occurred after doses of 150 to 800 mg in 3 of these young children (with dilated pupils in 1), and in a 2-year-old who also had semiconsciousness, pinpoint pupils, diminished tone, and elevated liver enzymes. Other children who ingested doses of 200 mg to 2.5 g showed no symptoms.
Among the adults, a 70-year-old man with a history of chronic obstructive airway disease died. Toxicological analysis showed acute flurbiprofen overdose and a blood ethanol concentration of 100 mg/dL. In the other cases, symptoms were as follows: coma and respiratory depression after 3-6 g; drowsiness, nausea and epigastric pain after 2.5-5 g; epigastric pain and dizziness after 3 g; headache and nausea after ≤ 2 g; agitation after 1.5 g; and drowsiness after 1.0 g. One patient, who took 200-400 mg flurbiprofen and 2.4 g fenoprofen, had disorientation and diplopia. Three adults had no symptoms after 3-5 g flurbiprofen.
Treatment of an overdose: the stomach should be emptied by vomiting or lavage, though little drug will likely be recovered if more than an hour has elapsed since ingestion. Supportive treatment should be instituted as necessary. Some patients have been given supplemental oral or intravenous fluids and required no other treatment.
In mice, the flurbiprofen LD50 was 750 mg/kg when administered orally and 200 mg/kg when administered intraperitoneally. The primary signs of toxicity were prostration, ataxia, loss of righting reflex, labored respiration, twitches, convulsions, CNS depression, and splayed hind limbs. In rats, the flurbiprofen LD50 was 160 mg/kg when administered orally and 400 mg/kg when administered intraperitoneally. The primary signs of toxicity were tremors, convulsions, labored respiration, and prostration. These were observed mostly in the intraperitoneal studies.
DOSAGE AND ADMINISTRATION
Flurbiprofen is administered orally.
Rheumatoid arthritis and osteoarthritis: Recommended starting dose is 200 to 300 mg total daily dose administered BID, TID, or QID. (Most experience in rheumatoid arthritis has been with TID or QID dosage.) The largest recommended single dose in a multiple-dose daily regimen is 100 mg. The dose should be tailored to each patient according to the severity of the symptoms and the response to therapy.
Although a few patients have received higher doses, doses above 300 mg per day are not recommended until more clinical experience with flurbiprofen is obtained.
HOW SUPPLIED
Flurbiprofen Tablets USP, 100 mg are round, blue, film-coated tablets debossed “93”-“711” available in bottles of 100 and 500.
Store at controlled room temperature, between 20° and 25°C (68° and 77°F) (see USP).
Dispense contents in a well-closed container as defined in the USP/NF, with a child-resistant closure (as required).
Manufactured By:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. E 6/2003
| Flurbiprofen (Flurbiprofen) | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
Revised: 01/2006