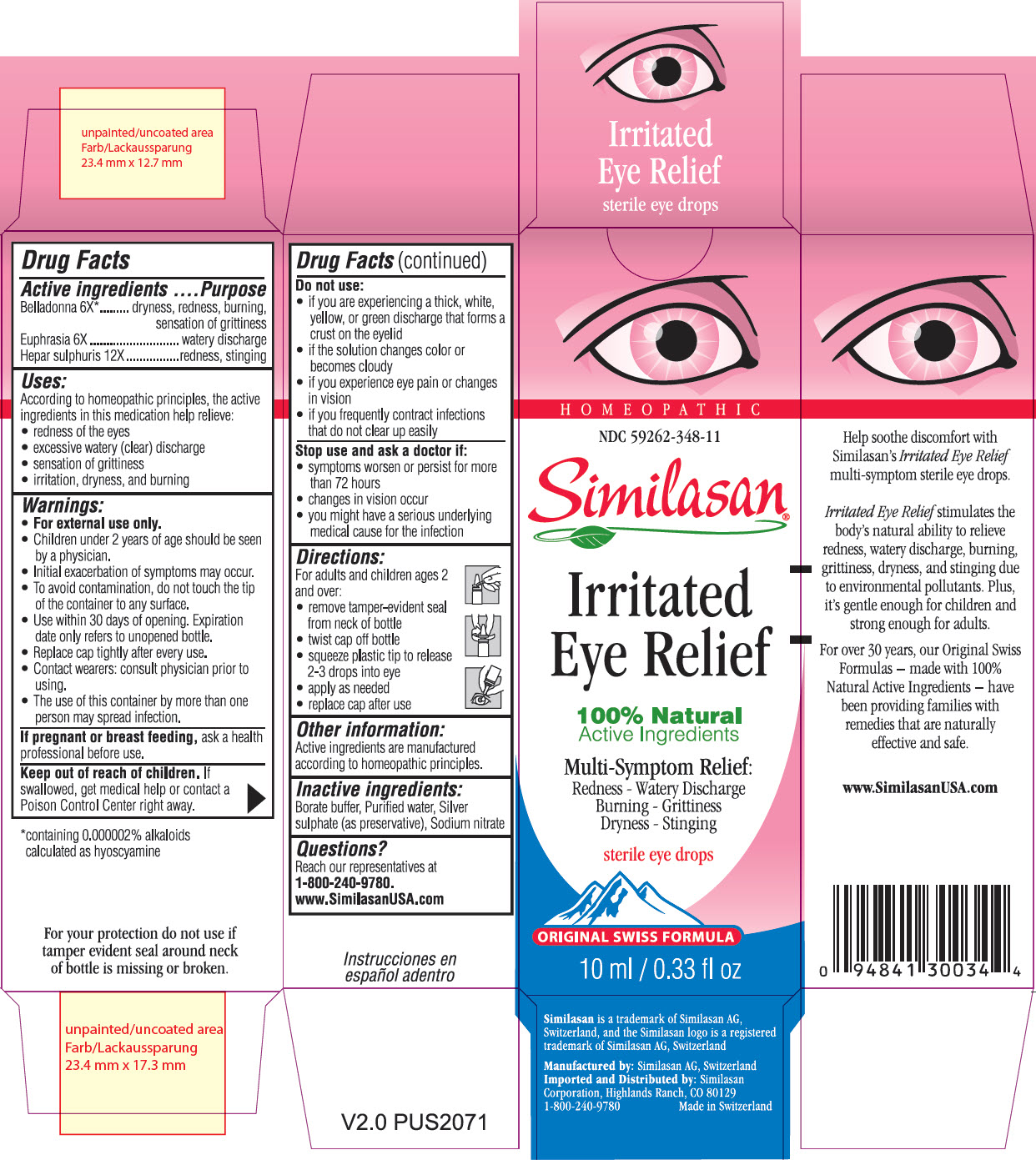
IRRITATED EYE RELIEF- atropa belladonna, euphrasia stricta and calcium sulfide solution/ drops
Similasan AG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Uses:
According to homeopathic principles, the active ingredients in this medication help relieve:
- redness of the eyes
- excessive watery (clear) discharge
- sensation of grittiness
- irritation, dryness, and burning
Warnings:
- For external use only.
- Children under 2 years of age should be seen by a physician.
- Initial exacerbation of symptoms may occur.
- To avoid contamination, do not touch the tip of the container to any surface.
- Use within 30 days of opening. Expiration date only refers to unopened bottle.
- Replace cap tightly after every use.
- Contact wearers: consult a physician prior to using.
- The use of this container by more than one person may spread infection.
Do Not Use:
- if you are experiencing a think, white, yellow, or green discharge that forms a crust on the eyelid
- if the solution changes color or becomes cloudy
- if you experience eye pain or changes in vision
- if you frequently contract infections that do not clear up easily
Stop use and ask a doctor if:
- symptoms worsen or persist for more than 72 hours
- changes in vision occur
- you might have a serious underlying medical cause for the infection
Directions:
For adults and children ages 2 and over:
- remove tamper-evident seal from neck of bottle
- twist cap off bottle
- squeeze plastic tip to release 2-3 drops into eye
- apply as needed
- replace cap after use
| IRRITATED EYE RELIEF
atropa belladonna and calcium sulfide and euphrasia stricta solution/ drops |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Similasan AG (481545754) |
Revised: 11/2011
Document Id: b55865f5-3347-4596-9737-f66db0b152fa
Set id: 971a3887-42d8-49d9-82a0-eca2fe59340b
Version: 21
Effective Time: 20111122
Similasan AG