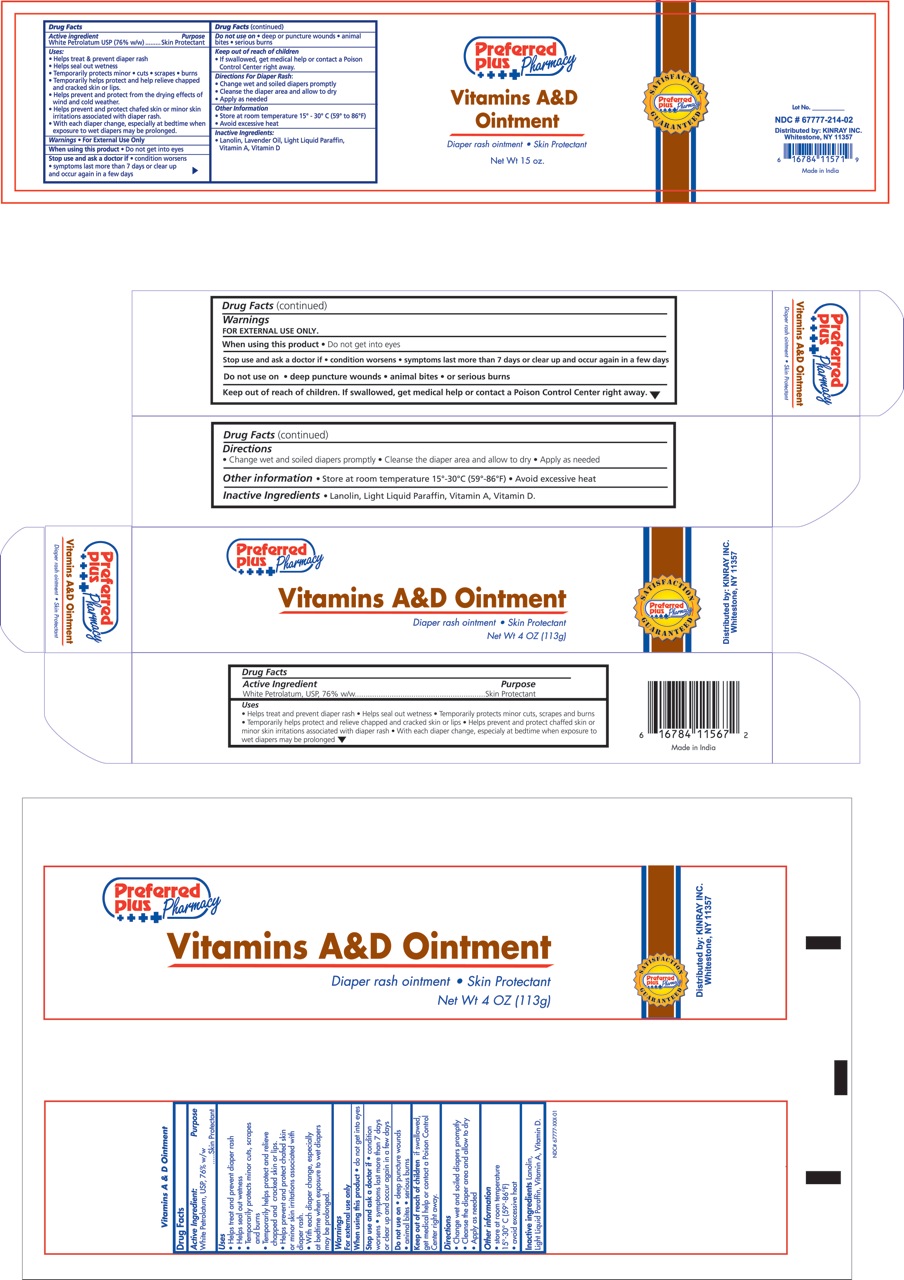
KINRAY VITAMIN A D- petrolatum ointment
Kinray Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Kinray Vitamin A D
Kinray Uses:
- Helps treat and prevent diaper rash
- Helps seal out wetness
- Temporarily protects minor * cuts * scrapes * burns
- Temporarily helps protect and help relieve chapped and cracked skin and lips
- Helps prevent and protect from the drying effects of wind and cold weather
- Helps prevent and protect chafed skin or minor skin irritations associated with diaper rash
- With each diaper change, especially at bedtime when exposure to wet diapers may be prolonged
Stop use and ask a doctor if:
- If condition worsens
- symptoms last more than 7 days or clear up and occur again in a few days
Keep out of reach of children
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions for diaper rash:
- Change wet and soiled diapers promptly
- Cleanse the diaper area and allow to dry
- Apply as needed
| KINRAY VITAMIN A D
petrolatum ointment |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Kinray Inc. (012574513) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Galentic Pharma India Private Limited | 918531450 | manufacture(63777-214) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Choice Laboratories | 650070394 | manufacture(63777-214) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amar Remedies Limited | 915839811 | manufacture(63777-214) | |
Revised: 11/2012
Document Id: 0e01c189-4410-4e3e-8e2f-d9ef2521f13f
Set id: 87c76eb4-309a-419a-8d43-5ef4a39d7f2a
Version: 16
Effective Time: 20121130
Kinray Inc.