DORAL
-
quazepam tablet
Physicians Total Care, Inc.
----------
DESCRIPTION
DORAL® (brand of quazepam) Tablets contain quazepam, a trifluoroethyl benzodiazepine hypnotic agent, having the chemical name 7-chloro-5- (o-fluoro-phenyl)-1,3-dihydro-1-(2,2,2-trifluoroethyl)-2H-1,4-benzodiazepine-2-thione and the following structural formula: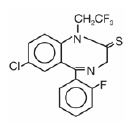
Quazepam has the empirical formula C17H11CIF4N2S, and a molecular weight of 386.8. It is a white crystalline compound, soluble in ethanol and insoluble in water. Each DORAL® Tablet contains either 7.5 or 15 mg of quazepam. The inactive ingredients for DORAL® Tablets 7.5 or 15 mg include cellulose, corn starch, FD&C Yellow No. 6 Al Lake, lactose, magnesium stearate, silicon dioxide, and sodium lauryl sulfate.
CLINICAL PHARMACOLOGY
Central nervous system agents of the 1,4-benzodiazepine class presumably exert their effects by binding to stereo-specific receptors at several sites within the central nervous system (CNS). Their exact mechanism of action is unknown.In a sleep laboratory study, DORAL® Tablets significantly decreased sleep latency and total wake time, and significantly increased total sleep time and percent sleep time, for one or more nights. Quazepam 15 mg was effective on the first night of administration. Sleep latency, total wake time and wake time after sleep onset were still decreased and percent sleep time was still increased for several nights after the drug was discontinued. Percent slow wave sleep was decreased, and REM sleep was essentially unchanged. No transient sleep disturbance, such as “rebound insomnia,” was observed after withdrawal of the drug in sleep laboratory studies in 12 patients using 15 mg doses.
In outpatient studies, DORAL® Tablets improved all subjective measures of sleep including sleep induction time, duration of sleep, number of nocturnal awakenings, occurrence of early morning awakening, and sleep quality. Some effects were evident on the first night of administration of DORAL® Tablets (sleep induction time, number of nocturnal awakenings, and duration of sleep). Residual medication effects (“hangover”) were minimal.
Quazepam is rapidly (absorption half-life of about 30 minutes) and well absorbed from the gastrointestinal tract. The peak plasma concentration of quazepam is approximately 20 ng/mL after a 15 mg dose and is obtained at about 2 hours. Quazepam, the active parent compound, is extensively metabolized in the liver; two of the plasma metabolites are 2-oxoquazepam and N-desalkyl-2-oxoquazepam. All three compounds show pharmacological central nervous system activity in animals.
Following administration of 14C-quazepam, approximately 31% of the dose appears in the urine and 23% in the feces over a five-day period; only trace amounts of unchanged drug are present in the urine.
The mean elimination half-life of quazepam and 2-oxoquazepam is 39 hours and that of N-desalkyl-2-oxoquazepam is 73 hours. Steady-state levels of quazepam and 2-oxoquazepam are attained by the seventh daily dose and that of N-desalkyl-2-oxoquazepam by the thirteenth daily dose.
The pharmacokinetics of quazepam and 2-oxoquazepam in geriatric subjects are comparable to those seen in young adults; as with desalkyl metabolites of other benzodiazepines, the elimination half-life of N-desalkyl-2-oxoquazepam in geriatric patients is about twice that of young adults.
The degree of plasma protein binding for quazepam and its two major metabolites is greater than 95%. The absorption, distribution, metabolism, and excretion of benzodiazepines may be altered in various disease states including alcoholism, impaired hepatic function, and impaired renal function.
The type and duration of hypnotic effects and the profile of unwanted effects during administration of benzodiazepine drugs may be influenced by the biologic half-life of administered drug and any active metabolites formed. When half-lives are long, drug or metabolites may accumulate during periods of nightly administration and be associated with impairments of cognitive and/or motor performance during waking hours; the possibility of interaction with other psychoactive drugs or alcohol will be enhanced. In contrast, if half-lives are short, drug and metabolites will be cleared before the next dose is ingested, and carry-over effects related to excessive sedation or CNS depression should be minimal or absent. However, during nightly use for an extended period, pharmacodynamic tolerance or adaptation to some effects of benzodiazepine hypnotics may develop. If the drug has a short half-life of elimination, it is possible that a relative deficiency of the drug or its active metabolites (i.e., in relationship to the receptor site) may occur at some point in the interval between each night’s use. This sequence of events may account for two clinical findings reported to occur after several weeks of nightly use of rapidly eliminated benzodiazepine hypnotics, namely, increased wakefulness during the last third of the night, and the appearance of increased signs of daytime anxiety in selected patients.
Quazepam crosses the placental barrier of mice. Quazepam, 2-oxoquazepam and N-desalkyl-2-oxoquazepam are present in breast milk of lactating women, but the total amount found in the milk represents only about 0.1% of the administered dose.
Drug-Drug Interactions (see also PRECAUTIONS: Drug Interactions):
In vitro inhibition studies conducted to assess the potential of quazepam to inhibit CYP2B6, CYP2C8 and CYP2E1 at relevant clinical Cmax concentrations (0.15 μM = 58 ng/mL) demonstrate quazepam is a CYP2B6 mechanism based inhibitor. Increased plasma concentrations of drugs that are substrates of CYP2B6 may result if co-administered with DORAL®. Quazepam does not inhibit CYP2C8 and CYP2E1.
INDICATIONS AND USAGE
DORAL® Tablets are indicated for the treatment of insomnia characterized by difficulty in falling asleep, frequent nocturnal awakenings, and/or early morning awakenings. The effectiveness of DORAL® has been established in placebo-controlled clinical studies of 5 nights duration in acute and chronic insomnia. The sustained effectiveness of DORAL® has been established in chronic insomnia in a sleep lab (polysomnographic) study of 28 nights duration.Because insomnia is often transient and intermittent, the prolonged administration of DORAL® Tablets is generally not necessary or recommended. Since insomnia may be a symptom of several other disorders, the possibility that the complaint may be related to a condition for which there is a more specific treatment should be considered.
CONTRAINDICATIONS
DORAL® Tablets are contraindicated in patients with known hypersensitivity to this drug or other benzodiazepines, and in patients with established or suspected sleep apnea.
Usage in Pregnancy: Benzodiazepines may cause fetal damage when administered during pregnancy. An increased risk of congenital malformations associated with the use of diazepam and chlordiazepoxide during the first trimester of pregnancy has been suggested in several studies. Transplacental distribution has resulted in neonatal CNS depression following the ingestion of therapeutic doses of a benzodiazepine hypnotic during the last weeks of pregnancy.
DORAL® Tablets are contraindicated in pregnancy because the potential risks outweigh the possible advantages of their use during this period. If there is a likelihood of the patient becoming pregnant while receiving DORAL®, she should be warned of the potential risk to the fetus. Patients should be instructed to discontinue the drug prior to becoming pregnant. The possibility that a woman of child-bearing potential may be pregnant at the time of institution of therapy should be considered. (see Pregnancy, Teratogenic Effects: Pregnancy Category X).
WARNINGS
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs. Because some of the important adverse effects of sedative-hypnotics appear to be dose related (see Precautions and Dosage and Administration), it is important to use the smallest possible effective dose, especially in the elderly.Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naive as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with sedative-hypnotics alone at therapeutic doses, the use of alcohol and other CNS depressants with sedative-hypnotics appears to increase the risk of such behaviors, as does the use of sedative-hypnotics at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of sedative-hypnotics should be strongly considered for patients who report a "sleep-driving" episode.
Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
Severe anaphylactic and anaphylactoid reactions
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including Doral. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis.
Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with Doral should not be rechallenged with the drug.
Patients receiving benzodiazepines should be cautioned about possible combined effects with alcohol and other CNS depressants. Also, caution patients that an additive effect may occur if alcoholic beverages are consumed during the day following the use of benzodiazepines for nighttime sedation. The potential for this interaction continues for several days following their discontinuance until serum levels of psychoactive metabolites have declined.
Patients should also be cautioned about engaging in hazardous occupations requiring complete mental alertness, such as operating machinery or driving a motor vehicle, after ingesting benzodiazepines, including potential impairment of the performance of such activities which may occur the day following ingestion.
Withdrawal symptoms of the type associated with sedatives/hypnotics (e.g., barbiturates, bromides, etc.) and alcohol have been reported after the discontinuation of benzodiazepines. While these symptoms have been more frequently reported after the discontinuation of excessive benzodiazepine doses, there have also been controlled studies demonstrating the occurrence of such symptoms after discontinuation of therapeutic doses of benzodiazepines, generally following prolonged use (but in some instances after periods as brief as 6 weeks). It is generally believed that the gradual reduction of dosage will diminish the occurrence of such symptoms (see DRUG ABUSE AND DEPENDENCE).
PRECAUTIONS
General:Impaired motor and/or cognitive performance attributable to the accumulation of benzodiazepines and their active metabolites following several days of repeated use at their recommended doses is a concern in certain vulnerable patients (e.g., those especially sensitive to the effects of benzodiazepines or those with a reduced capacity to metabolize and eliminate them). Consequently, elderly or debilitated patients and those with impaired renal or hepatic function should be cautioned about the risk and advised to monitor themselves for signs of excessive sedation or impaired coordination.
The possibility of respiratory depression in patients with chronic pulmonary insufficiency should be considered.
When benzodiazepines are administered to depressed patients, there is a risk that the signs and symptoms of depression may be intensified. Consequently, appropriate precautions (e.g., limiting the total prescription size and increased monitoring for suicidal ideation) should be considered.
Information for Patients:
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with Doral and should counsel them in its appropriate use. A patient Medication Guide is available for Doral. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking Doral.
“Sleep-Driving" and other complex behaviors:
There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when sedative-hypnotics are taken with alcohol or other central nervous system depressants (see WARNINGS). Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
It is also suggested that physicians discuss the following information with patients. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
- Inform your physician about any alcohol consumption and medicine you are taking now, including drugs you may buy without a prescription. Alcohol should generally not be used during treatment with hypnotics.
- Inform your physician if you are planning to become pregnant, if you are pregnant, or if you become pregnant while you are taking this medicine.
- Inform your physician if you are nursing.
- Until you experience how this medicine affects you, do not drive a car or operate potentially dangerous machinery, etc.
- Benzodiazepines may cause daytime sedation, which may persist for several days following drug discontinuation.
- Patients should be told not to increase the dose on their own and should inform their physician if they believe the drug “does not work anymore”.
- If benzodiazepines are taken on a prolonged and regular basis (even for periods as brief as 6 weeks), patients should be advised not to stop taking them abruptly or to decrease the dose without consulting their physician, because withdrawal symptoms may occur.
Laboratory Tests:
Laboratory tests are not ordinarily required in otherwise healthy patients when quazepam is used as recommended.
Drug Interactions:
The benzodiazepines, including DORAL® Tablets, produce additive CNS depressant effects when co-administered with psychotropic medications, anticonvulsants, antihistaminics, ethanol, and other drugs which produce CNS depression.
Quazepam is a mechanism based inhibitor of CYP2B6 based on an in vitro study. However, the in vivo extrapolation of this is unknown. It may be possible that co-administration of DORAL® and drugs primarily metabolized by CYP2B6 (e.g., efavirenz and bupropion) may result in increased plasma concentrations of these drugs resulting in an increase in adverse events (e.g., CNS toxicities associated with efavirenz and precipitation of seizures with bupropion). Patients taking medications that are CYP2B6 substrates with DORAL® should be monitored closely for adverse reactions associated with these medications. If adverse events are observed, clinicians may consider the discontinuation of DORAL® and the selection of an alternative anxiolytic agent.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Quazepam showed no evidence of carcinogenicity or other significant pathology in oral oncogenicity studies in mice and hamsters.
Quazepam was tested for mutagenicity using the L5178Y TK +/-Mouse Lymphoma Mutagenesis Assay and the Ames Test. The L5178Y TK +/-Assay was equivocal and the Ames Test did not show mutagenic activity.
Reproduction studies in mice conducted with quazepam at doses equal to 60 and 180 times the human dose of 15 mg, and with diazepam at 67 times the human dose, produced slight reductions in the pregnancy rate. Similar reduction in pregnancy rates have been reported in mice dosed with other benzodiazepines, and is believed to be related to the sedative effects of these drugs at high doses.
Pregnancy, Teratogenic Effects:
Pregnancy Category X (See CONTRAINDICATIONS, Usage in Pregnancy) Reproduction studies of quazepam in mice at doses up to 400 times the human dose revealed no major drug-related malformations. Minor developmental variations that occurred were delayed ossification of the sternum, vertebrae, distal phalanges and supraoccipital bones, at doses of 66 and 400 times the human dose. Studies with diazepam at 200 times the human dose showed a similar or greater incidence than quazepam. A reproduction study of quazepam in New Zealand rabbits at doses up to 134 times the human dose demonstrated no effect on fetal morphology or development of offspring.
Nonteratogenic Effects:
The child born of a mother who is taking benzodiazepines may be at some risk of withdrawal symptoms from the drug during the postnatal period. Neonatal flaccidity has been reported in children born of mothers who had been receiving benzodiazepines.
Labor and Delivery:
DORAL® Tablets have no established use in labor or delivery.
Nursing Mothers:
Quazepam and its metabolites are excreted in the milk of lactating women. Therefore, administration of DORAL® Tablets to nursing women is not recommended.
Pediatric Use:
Safety and effectiveness in children below the age of 18 years have not been established.
Geriatric Use:
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Questcor Pharmaceuticals, Inc. at 1-800-465-9217 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.Adverse events most frequently encountered in patients treated with quazepam are drowsiness and headache.
Accurate estimates of the incidence of adverse events associated with the use of any drug are difficult to obtain. Estimates are influenced by drug dose, detection technique, setting, physician judgments, etc. Consequently, the table below is presented solely to indicate the relative frequency of adverse events reported in representative controlled clinical studies conducted to evaluate the safety and efficacy of quazepam. The figures cited cannot be used to predict precisely the incidence of such events in the course of usual medical practice. These figures, also, cannot be compared with those obtained from other clinical studies involving related drug products and placebo.
The figures cited below are estimates of untoward clinical event incidences of 1% or greater among subjects who participated in the relatively short-duration, placebo-controlled clinical trials of quazepam.
|
| DORAL * | PLACEBO |
| NUMBER OF PATIENTS | 267 | 268 |
| % OF PATIENTS REPORTING |
|
|
| Central Nervous System |
|
|
| Daytime Drowsiness | 12.0 | 3.3 |
| Headache | 4.5 | 2.2 |
| Fatigue | 1.9 | 0 |
| Dizziness | 1.5 | <1 |
| Autonomic neervous System |
|
|
| Dry Mouth | 1.5 | <1 |
| Gastrointestinal System |
|
|
| Dyspepsia | 1.1 | <1 |
* DORAL 15 mg
The following incidences of laboratory abnormalities occurred at a rate of 1% or greater in patients receiving quazepam and the corresponding placebo group. None of these changes were considered to be of physiological significance.
|
|
| DORAL |
| PLACEBO |
| NUMBER OF PATIENTS |
| 234 |
| 244 |
| % OF PATIENTS REPORTING | Low | High | Low | High |
| Hematology |
|
|
|
|
| Hemoglobin | 1.4 | 0. | 1.2 | 0 |
| Hematocrit | 1.5 | 0 | 1.7 | 0 |
| Lymphocyte | 1.3 | 1.6 | 1.2 | 1.9 |
| Eosinophil | * | 1.5 | * | 1.3 |
| SEG | 1.1 | * | 1.6 | * |
| Monocyte | * | 1.1 | * | * |
| Blood Chemistry |
|
|
|
|
| Glucose | * | * | * | 1.2 |
| SGOT | * | 1.3 | * | 1.1 |
| Urinalysis |
|
|
|
|
| Specific Gravity | * | * | * | 1.1 |
| WBC | 0 | 2.6 | 0 | 3.0 |
| RBC | 0 | * | 0 | 1.1 |
| Epithelial Cells | 0 | 2.5 | 0 | 3.2 |
| Crystals | 0 | * | 0 | 1.0 |
abnormalities in the following laboratory tests were observed in less than 1% of
the patients evaluated: WBC count, total protein, albumin, BUN, creatinine,
total bilirubin, alkaline phosphatase, and SGPT.
The following additional events occurred among individuals receiving quazepam at doses equivalent to or greater than those recommended during its clinical testing and development. There is no way to establish whether or not the administration of DORAL® caused these events.
Hypokinesia, ataxia, confusion, incoordination, hyperkinesia, speech disorder, and tremor were reported.
Also, depression, nervousness, agitation, amnesia, anorexia, anxiety, apathy, euphoria, impotence, decreased libido, paranoid reaction, nightmares, abnormal thinking, abnormal taste perception, abnormal vision, and cataract were reported.
Also reported were urinary incontinence, palpitations, nausea, constipation, diarrhea, abdominal pain, pruritus, rash, asthenia, and malaise.
The following list provides an overview of adverse experiences that have been reported and are considered to be reasonably related to the administration of benzodiazepines: incontinence, slurred speech, urinary retention, jaundice, dysarthria, dystonia, changes in libido, irritability, and menstrual irregularities.
As with all benzodiazepines, paradoxical reactions such as stimulation, agitation, increased muscle spasticity, sleep disturbances, hallucinations, and other adverse behavioral effects may occur in rare instances and in a random fashion. Should these occur, use of the drug should be discontinued.
There have been reports of withdrawal signs and symptoms of the type associated with withdrawal from CNS depressant drugs following the rapid decrease or the abrupt discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE).
DRUG ABUSE AND DEPENDENCE
Controlled Substance:
DORAL® is a controlled substance under the Controlled Substances Act and has been assigned by the Drug Enforcement Administration to Schedule IV.
Abuse and Dependence:
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of the drug for non-medical purposes, often in combination with other psychoactive substances. Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug and/or administration of an antagonist. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both the desired and undesired effects of drugs and may develop at different rates for different effects.
Addiction is a primary, chronic, neurobiological disease with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (e.g., convulsions, tremor, abdominal and muscle cramps, vomiting, and sweating), have occurred following abrupt discontinuance of benzodiazepines. The more severe withdrawal symptoms have usually been limited to those patients who received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving quazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
OVERDOSAGE
Manifestations of overdosage seen with other benzodiazepines include somnolence, confusion, and coma. In the event that an overdose occurs, the following is the recommended treatment. Respiration, pulse, and blood pressure should be monitored, as in all cases of drug overdosage. General supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered and an adequate airway maintained. Hypotension may be treated with the use of norepinephrine bitartrate or metaraminol bitartrate. Dialysis is of limited value. Animal experiments suggest that forced diuresis or hemodialysis are probably of little value in treating overdosage. As with the management of intentional overdosing with any drug, it should be borne in mind that multiple agents may have been ingested.The oral LD50 in mice was greater than 5000 mg/kg.
DOSAGE AND ADMINISTRATION
Adults:Initiate therapy at 15 mg until individual responses are determined. In some patients, the dose may then be reduced to 7.5 mg.
Elderly and Debilitated Patients:
Because the elderly and debilitated may be more sensitive to benzodiazepines, attempts to reduce the nightly dosage after the first 1-2 nights of therapy are suggested.
Geriatric Patients:
A double-blind controlled sleep laboratory study (N=30) compared the effects of quazepam 7.5 mg and 15 mg to that of placebo over a period of 7 days. Both the 7.5 mg and 15 mg doses appeared to be well tolerated. Caution must be used in interpreting this data due to the small size of the study; therefore, initiate therapy in geriatric patients at 7.5 mg. If not effective after 1-2 nights, the dosage may be increased to 15 mg.
HOW SUPPLIED
DORAL® Tablets, 15 mg, unscored, capsule-shaped, light orange, slightly white speckled tablets, impressed with the product identification number 15 on one side of the tablet, and the product name (DORAL) on the other.
15 mg Bottles of 30 NDC 54868-2826-1
Store DORAL® Tablets at controlled room temperature 20°-25°C (68°-77°F).
To report SUSPECTED ADVERSE REACTIONS, contact Questcor Pharmaceuticals, Inc. at 1-800-465-9217 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured for:
Questcor Pharmaceuticals, Inc.
Union City, CA 94587 USA
Manufactured by:
Meda Pharmaceuticals
Meda Pharmaceuticals Inc.
Somerset, NJ 08873-4120
Under license from Baker Norton Pharmaceuticals Inc.
Printed in U.S.A.
PM-267-03 08/11
Distributed and Repackaged by:
Physicians Total Care, inc.
Tulsa, Oklahoma 74146
MEDICATION GUIDE
SEDATIVE-HYPNOTIC
TABLETS / CAPSULES C-IV
Read this Medication Guide before you start taking a SEDATIVEHYPNOTIC and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your medical condition or treatment. You and your doctor should talk about the SEDATIVE-HYPNOTIC when you start taking it and at regular checkups.
What is the most important information I should know about SEDATIVE-HYPNOTICS?
After taking a SEDATIVE-HYPNOTIC, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing. The next morning, you may not remember that you did anything during the night. You have a higher chance for doing these activities if you drink alcohol or take other medicines that make you sleepy with a SEDATIVEHYPNOTIC.
Reported activities include:
- driving a car (“sleep-driving”)
- making and eating food
- talking on the phone
- having sex
- sleep-walking
Important:
1. Take SEDATIVE-HYPNOTICS exactly as prescribed
- Do not take more SEDATIVE-HYPNOTICS than prescribed.
- Take the SEDATIVE-HYPNOTIC right before you get in bed,not sooner.
2. Do not take SEDATIVE-HYPNOTICS if you:
- drink alcohol
- take other medicines that can make you sleepy. Talk to your doctor about all of your medicines. Your doctor will tell you if you can take SEDATIVE-HYPNOTICS with your other medicines
- cannot get a full night’s sleep
3. Call your doctor right away if you find out that you have done any of the above activities after taking the SEDATIVEHYPNOTIC.
What are SEDATIVE-HYPNOTICS?
SEDATIVE-HYPNOTICS are sleep medicines. SEDATIVEHYPNOTICS are used in adults for the short-term treatment of the symptom of trouble falling asleep from insomnia. SEDATIVEHYPNOTICS do not treat other symptoms of insomnia which include waking up too early in the morning and waking up often during the night.
SEDATIVE-HYPNOTICS are not for children.
SEDATIVE-HYPNOTICS are federally controlled substances (C-IV) because they can be abused or lead to dependence. Keep SEDATIVE-HYPNOTICS in a safe place to prevent misuse and abuse. Selling or giving away SEDATIVEHYPNOTICS may harm others, and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
Who should not take SEDATIVE-HYPNOTICS?
Do not take SEDATIVE-HYPNOTICS if you are allergic to anything in it. See the end of this Medication Guide for a complete list of ingredients in DORAL.
SEDATIVE-HYPNOTICS may not be right for you. Before starting SEDATIVE-HYPNOTICS, tell your doctor about all of your health conditions, including if you:
- have a history of depression, mental illness, or suicidal thoughts
- have a history of drug or alcohol abuse or addiction
- have kidney or liver disease
- have a lung disease or breathing problems
- are pregnant, planning to become pregnant, or breastfeeding
Tell your doctor about all of the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Medicines can interact, sometimes causing side effects. Do not take SEDATIVE-HYPNOTICS with other medicines that can make you sleepy.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
How should I take SEDATIVE-HYPNOTICS?
- Take SEDATIVE-HYPNOTICS exactly as prescribed. Do not take more SEDATIVE-HYPNOTIC than prescribed for you.
- Take SEDATIVE-HYPNOTICS right before you get into bed. Or you can take the SEDATIVE-HYPNOTIC after youhave been in bed and have trouble falling asleep.
- Do not take SEDATIVE-HYPNOTICS with or right after a meal.
- Do not take SEDATIVE-HYPNOTICS unless you are able to get a full night’s sleep before you must be active again.
- Call your healthcare provider if your insomnia worsens or is not better within 7 to 10 days. This may mean that there is another condition causing your sleep problem.
- If you take too much SEDATIVE-HYPNOTIC or overdose, call your doctor or poison control center right away, or get emergency treatment.
What are the possible side effects of SEDATIVE-HYPNOTICS?
Serious side effects of SEDATIVE-HYPNOTICS include:
- getting out of bed while not being fully awake and do an activity that you do not know you are doing. (See “What is the most important information I should know about SEDATIVE-HYPNOTICS?”)
- abnormal thoughts and behavior. Symptoms include more outgoing or aggressive behavior than normal, confusion, agitation, hallucinations, worsening of depression, and suicidal thoughts or actions.
- memory loss
- anxiety
- severe allergic reactions. Symptoms include swelling of the tongue or throat, trouble breathing, and nausea and vomiting. Get emergency medical help if you get these symptoms after taking SEDATIVE-HYPNOTICS.
Call your doctor right away if you have any of the above side effects or any other side effects that worry you while using the SEDATIVE-HYPNOTIC.
Common side effects of SEDATIVE-HYPNOTICS include:
- drowsiness
- headache
- fatigue
- dizziness
- dry mouth
- upset stomach
- You may still feel drowsy the next day after taking the SEDATIVE-HYPNOTIC. Do not drive or do other dangerous activities after taking the SEDATIVE-HYPNOTIC until you feel fully awake.
- You may have withdrawal symptoms for 1 to 2 days when you stop taking the SEDATIVE-HYPNOTIC. Withdrawal symptoms include trouble sleeping, unpleasant feelings, stomach and muscle cramps, vomiting, sweating, shakiness, and seizures.
These are not all the side effects of SEDATIVE-HYPNOTICS.
Ask your doctor or pharmacist for more information.
How should I store SEDATIVE-HYPNOTICS?
- Store SEDATIVE-HYPNOTICS at room temperature between 68° and 77° F (20° to 25°C).
- Protect from light.
- Keep SEDATIVE-HYPNOTICS and all medicines out of the reach of children.
General Information about SEDATIVE-HYPNOTICS
- Medicines are sometimes prescribed for purposes not mentioned in a Medication Guide.
- Do not use the SEDATIVE-HYPNOTIC for a condition for which it was not prescribed.
- Do not give the SEDATIVE-HYPNOTIC to other people, even if they have the same condition. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about SEDATIVE-HYPNOTICS. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about the SEDATIVE-HYPNOTIC that was written for healthcare professionals.
If you would like more information, contact Questcor Pharmaceuticals at 1-800-411-3065 or visit http://www.doralforsleep.com.
What are the ingredients in the SEDATIVE-HYPNOTIC?
Active Ingredient: quazepam
Inactive Ingredients: cellulose, corn starch, FD&C Yellow No. 6 Al Lake, lactose, magnesium stearate, silicon dioxide, and sodium lauryl sulfate.
Rx only
Distributed by:
Questcor Pharmaceuticals, Inc.
Union City, CA 94587 USA
This Medication Guide has been approved by the U.S. Food and Drug Administration.
IS-1500-01 Rev. 07/07
IS-1500-01
| DORAL
quazepam tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA018708 | 03/02/1994 | 06/30/2002 |
| Labeler - Physicians Total Care, Inc. (194123980) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Physicians Total Care, Inc. | 194123980 | relabel, repack | |
Revised: 10/2012 Physicians Total Care, Inc.