NYSTATIN
-
nystatin suspension
Taro Pharmaceuticals U.S.A., Inc.
----------
Nystatin
Oral Suspension,
USP
Rx only
DESCRIPTION
Nystatin is an antimycotic polyene antibiotic obtained from Streptomyces noursei. Structural formula:
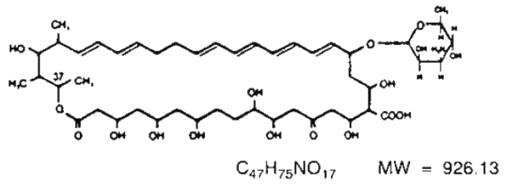
Nystatin Oral Suspension, for oral administration, is cherry/mint flavored, containing 100,000 USP Nystatin Units per mL. Inactive ingredients: alcohol (≤ 1% v/v), 49.8% (w/v) sucrose, purified water, glycerin, sodium citrate, magnesium aluminum silicate, flavors, saccharin sodium, xanthan gum, benzaldehyde, edetate calcium disodium, methylparaben and propylparaben.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Gastrointestinal absorption of nystatin is insignificant. Most orally administered nystatin is passed unchanged in the stool. In patients with renal insufficiency receiving oral therapy with conventional dosage forms, significant plasma concentrations of nystatin may occasionally occur.
Microbiology
Nystatin is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi. Candida albicans demonstrates no significant resistance to nystatin in vitro on repeated subculture in increasing levels of nystatin; other Candida species become quite resistant. Generally, resistance does not develop in vivo. Nystatin acts by binding to sterols in the cell membrane of susceptible Candida species with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
INDICATIONS AND USAGE
Nystatin Oral Suspension is indicated for the treatment of candidiasis in the oral cavity.
CONTRAINDICATIONS
The preparation is contraindicated in patients with a history of hypersensitivity to any of its components.
PRECAUTIONS
General
This medication is not to be used for the treatment of systemic mycoses. Discontinue treatment if sensitization or irritation is reported during use.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential. There also have been no studies to determine mutagenicity or whether this medication affects fertility in males or females.
Pregnancy
Teratogenic Effects Category C
Animal reproduction studies have not been conducted with nystatin oral suspension. It is also not known whether nystatin oral suspension can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Nystatin oral suspension should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether nystatin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nystatin is administered to a nursing woman.
Pediatric Use
See DOSAGE AND ADMINISTRATION.
ADVERSE REACTIONS
Nystatin is well tolerated even with prolonged therapy. Oral irritation and sensitization have been reported. (See PRECAUTIONS: General).
Gastrointestinal: Diarrhea (including one case of bloody diarrhea), nausea, vomiting, gastrointestinal upset/disturbances.
Dermatologic: Rash, including urticaria has been reported rarely. Stevens-Johnson syndrome has been reported very rarely.
Other: Tachycardia, bronchospasm, facial swelling, and non-specific myalgia have also been rarely reported.
OVERDOSAGE
Oral doses of nystatin in excess of five million units daily have caused nausea and gastrointestinal upset. There have been no reports of serious toxic effects of superinfections (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
DOSAGE AND ADMINISTRATION
INFANTS
2 mL (200, 000 units) four times daily (in infants and young children, use dropper to place one-half of dose in each side of mouth and avoid feeding for 5 to 10 minutes).
NOTE: Limited clinical studies in premature and low birth weight infants indicate that 1 mL four times daily is effective.
CHILDREN AND ADULTS
4-6 mL (400,000 to 600,000 units) four times daily (one-half of dose in each side of mouth). The preparation should be retained in the mouth as long as possible before swallowing.
Continue treatment for at least 48 hours after perioral symptoms have disappeared and cultures demonstrate eradication of Candida albicans.
HOW SUPPLIED
Nystatin Oral Suspension, USP, 100,000 USP
Nystatin U/mL, is available as a cherry-mint flavored, light creamy yellow, ready-to-use suspension in:
2 fl oz (60 mL) bottles with 0.5 mL, 1 mL, 1.5 mL, 2 mL calibrated dropper
NDC 51672-4117-4
1 Pint (473 mL) bottles
NDC 51672-4117-9
1 Gallon (3785 mL) bottles (For repackaging only)
NDC 51672-4117-0
Storage
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature]. Protect from freezing.
PHARMACIST: Dispense in a tight, light-resistant container as defined in USP.
Manufactured by: Taro Pharmaceutical Industries Ltd.,
Haifa Bay, Israel 26110
Distributed by: Taro Pharmaceuticals U.S.A., Inc.,
Hawthorne, N.Y. 10532
Issued: February, 2006
70564-0206-0
| NYSTATIN
nystatin suspension |
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Revised: 09/2008Taro Pharmaceuticals U.S.A., Inc.