FOLLISTIM AQ
-
follitropin beta injection, solution
Organon USA, Inc.
----------
DESCRIPTION
Follistim® AQ (follitropin beta injection) contains human follicle-stimulating hormone (hFSH), a glycoprotein hormone which is manufactured by recombinant DNA (rDNA) technology. The active drug substance, follitropin beta, has a dimeric structure containing two glycoprotein subunits (alpha and beta). Both the 92 amino acid alpha-chain and the 111 amino acid beta-chain have complex heterogeneous structures arising from two N-linked oligosaccharide chains. Follitropin beta is synthesized in a Chinese hamster ovary (CHO) cell line that has been transfected with a plasmid containing the two subunit DNA sequences encoding for hFSH. The purification process results in a highly purified preparation with a consistent hFSH isoform profile and high specific activity1. The biological activity is determined by measuring the increase in ovary weight in female rats. The intrinsic luteinizing hormone (LH) activity in follitropin beta is less than 1 IU per 40,000 IU FSH. The compound is considered to contain no LH activity.
The amino acid sequence and tertiary structure of the product are indistinguishable from that of human follicle-stimulating hormone (hFSH) of urinary source. Also, based on available data derived from physio-chemical tests and bioassay, follitropin beta and follitropin alfa, another recombinant follicle-stimulating hormone product, are indistinguishable.
Follistim® AQ is presented as a sterile aqueous solution intended for SUBCUTANEOUS or INTRAMUSCULAR administration. Each single-use vial of Follistim® AQ contains the following per 0.5 mL: 75 IU or 150 IU of FSH activity; 25 mg sucrose, NF; 7.35 mg sodium citrate (dihydrate), USP; 0.25 mg L-methionine, USP; 0.1 mg polysorbate 20, NF; and water for injection, USP. Hydrochloric acid, NF and/or sodium hydroxide, NF are used to adjust the pH to 7.
The recombinant protein in Follistim® AQ has been standardized for FSH in vivo bioactivity in terms of the First International Reference Preparation for human menopausal gonadotropins (code 70/45), issued by the World Health Organization Expert Committee on Biological Standardization (1982). Under current storage conditions, Follistim® AQ may contain up to 11% of oxidized follitropin beta.
In clinical trials with Follistim®, serum antibodies to FSH or anti-CHO cell derived proteins were not detected in any of the treated patients after exposure to Follistim® for up to three cycles.
Therapeutic Class: Infertility.
- 1
- As determined by the Ph. Eur. test for FSH in vivo bioactivity and on the basis of the molar extinction coefficient at 277 nm (εs:mg-1cm-1) = 1.066.
CLINICAL PHARMACOLOGY
Follicle-stimulating hormone (FSH), the active component in Follistim® AQ (follitropin beta injection), is required for normal follicular growth, maturation, and gonadal steroid production. In women, the level of FSH is critical for the onset and duration of follicular development, and consequently for the timing and number of follicles reaching maturity. Follistim® AQ stimulates ovarian follicular growth in women who do not have primary ovarian failure. In order to effect the final phase of follicle maturation, resumption of meiosis and rupture of the follicle in the absence of an endogenous LH surge, human chorionic gonadotropin (hCG) must be given following treatment with Follistim® AQ when patient monitoring indicates appropriate follicular development parameters have been reached.
Pharmacokinetics
Exposures of follitropin beta from Follistim® AQ and Follistim® are expected to be equivalent.
Absorption
The bioavailability of Follistim® following subcutaneous and intramuscular administration was investigated in healthy, pituitary-suppressed, female subjects given a single 300 IU dose. After subcutaneous or intramuscular injection the apparent dose absorbed was 77.8% and 76.4%, respectively.
The subcutaneous (455.6 ± 141.4 IU*h/L) and intramuscular (445.7 ± 135.7 IU*h/L) routes of administration were equivalent with respect to area under the curve (AUC) in healthy, pituitary-suppressed, female subjects given a single 300 IU dose. However, equivalence could not be established for peak serum FSH levels (Cmax) between the subcutaneous (5.41 ± 0.72 IU/L) and intramuscular (6.86 ± 2.90 IU/L) routes of administration.
The pharmacokinetics and pharmacodynamics of a single, intramuscular dose (300 IU) of Follistim® were also investigated in a group (n=8) of gonadotropin-deficient, but otherwise healthy women. In these women, FSH (mean ± SD) AUC was 339 ± 105 IU*h/L, Cmax was 4.3 ± 1.7 IU/L. Cmax occurred at approximately 27 ± 5.4 hours after intramuscular administration.
A multiple, dose proportionality, pharmacokinetic study of Follistim® was completed in healthy, pituitary-suppressed, female subjects given intramuscular doses of 75, 150, or 225 IU for 7 days. Steady-state blood concentrations of FSH were reached with all doses after 4 days of treatment based on the minimum concentrations of FSH just prior to dosing (Cmin). Peak blood concentrations with the 75, 150, and 225 IU dose were 4.65 ± 1.49 IU/L, 9.46 ± 2.57 IU/L and 11.30 ± 1.77 IU/L, respectively.
A multiple, dose proportionality, pharmacokinetic study of Follistim® was completed in healthy, pituitary-suppressed, female subjects given subcutaneous doses of 75, 150, or 225 IU for 7 days. Steady-state blood concentrations of FSH were reached with all doses after 5 days of treatment based on the minimum concentrations of FSH just prior to dosing (Cmin). Peak blood concentrations with the 75, 150, and 225 IU dose were 4.30 ± 0.60 IU/L, 8.51 ± 1.16 IU/L and 13.92 ± 1.81 IU/L, respectively.
Distribution
The volume of distribution of Follistim® in healthy, pituitary-suppressed, female subjects following intravenous administration of a 300 IU dose was approximately 8 L.
Metabolism
The recombinant FSH in Follistim® AQ is biochemically very similar to urinary FSH and it is therefore anticipated that it is metabolized in the same manner.
Elimination
The elimination half-life (t1/2) following a single intramuscular dose (300 IU) of Follistim® in female subjects was 43.9 ± 14.1 hours (mean ± SD). The elimination half-life following a 7-day intramuscular treatment with 75, 150, or 225 IU was 26.9 ± 7.8 hours (mean ± SD), 30.1 ± 6.2 and 28.9 ± 6.5, respectively.
Special Populations
The effect of body weight on the pharmacokinetics of Follistim® was evaluated in a group of European and Japanese women who were significantly different in terms of body weight. The European subjects had a body weight of (mean ± SD) 67.4 ± 13.5 kg and the Japanese subjects were 46.8 ± 11.6 kg. Following a single intramuscular dose of 300 IU of Follistim®, the AUC (IU*h/L) was significantly smaller in European subjects (339 ± 105) than in Japanese subjects (544 ± 201). However, clearance per kg of body weight was essentially the same for the respective groups (0.014 and 0.013 1*h-1kg-1).
The pharmacokinetics of Follistim® have not been determined in special populations such as geriatric, pediatric, renally impaired, and hepatically impaired patients.
Clinical Studies
The efficacy of Follistim® was established in four controlled, clinical studies [three studies for Assisted Reproductive Technologies (ART) and one study for Ovulation Induction], three of which are described below. In these comparative studies, there were no clinically significant differences between treatment groups in study outcomes.
Assisted Reproductive Technologies (ART)
Follistim® was studied in a randomized, assessor-blind, group comparative, multicenter safety and efficacy study of 981 infertile women treated for one cycle with in vitro fertilization with Follistim® or urofollitropin after pituitary suppression with a GnRH agonist. The results with Follistim® are summarized in Table 1.
| Parameter | Follistim® (n=585) |
|
|
| Total number of oocytes recovered | 10.9 |
| Ongoing† pregnancy rate/attempt‡ | 22.2% |
| Ongoing† pregnancy rate/transfer‡§ | 26.0% |
Follistim® was also evaluated in a randomized, assessor-blind, group comparative, single center safety and efficacy study in 89 infertile women treated with in vitro fertilization with Follistim® or menotropins without pituitary suppression with a GnRH agonist. The results with Follistim® are summarized in Table 2.
| Parameter | Follistim® (n=54) |
|
|
| Total number of oocytes recovered | 9.9 |
| Ongoing† pregnancy rate/attempt‡ | 22.2% |
| Ongoing† pregnancy rate/transfer‡§ | 30.8% |
Ovulation Induction
Results from a randomized, assessor-blind, group comparative, multicenter safety and efficacy study of Follistim® in 172 chronic anovulatory women who failed to ovulate and/or conceive during clomiphene citrate treatment are summarized in Tables 3 and 4.
| Cycle | Follistim® (n=105) |
| First treatment cycle | 72% |
| Second treatment cycle | 82% |
| Third treatment cycle | 85% |
| Cycle | Follistim® (n=105) |
| First treatment cycle | 14% |
| Second treatment cycle | 19% |
| Third treatment cycle | 23% |
INDICATIONS AND USAGE
Follistim® AQ (follitropin beta injection) is indicated for the development of multiple follicles in ovulatory patients participating in an Assisted Reproductive Technology (ART) program. Follistim® AQ is also indicated for the induction of ovulation and pregnancy in anovulatory infertile patients in whom the cause of infertility is functional and not due to primary ovarian failure.
Selection of Patients
Before treatment with Follistim® AQ is instituted:
- A thorough gynecologic and endocrinologic evaluation of the patient must be performed. The evaluation should include a hysterosalpingogram (to rule out uterine and tubal pathology) and documentation of anovulation by means of reviewing a patient’s history, performing a physical examination, determining serum hormonal levels as indicated, and optionally performing an endometrial biopsy. Patients with tubal pathology should receive Follistim® AQ only if enrolled in an ART program.
- Primary ovarian failure should be excluded by the determination of circulating gonadotropin levels.
- Careful examination should be made to rule out early pregnancy.
- Evaluation of the partner’s fertility potential should be included in the workup procedure.
CONTRAINDICATIONS
Follistim® AQ (follitropin beta injection) is contraindicated in women who exhibit:
- Prior hypersensitivity to recombinant hFSH products.
- High levels of FSH indicating primary ovarian failure.
- Uncontrolled thyroid or adrenal dysfunction.
- Tumor of the ovary, breast, uterus, hypothalamus, or pituitary gland.
- Pregnancy.
- Heavy or irregular vaginal bleeding of undetermined origin.
- Ovarian cysts or enlargement not due to polycystic ovary syndrome (PCOS).
- Hypersensitivity reactions to streptomycin or neomycin. Follistim® AQ may contain traces of these antibiotics and may cause hypersensitivity reactions in susceptible persons.
WARNINGS
Follistim® AQ (follitropin beta injection) should be used only by physicians who are experienced in infertility treatment. Follistim® AQ is a potent gonadotropic substance capable of causing Ovarian Hyperstimulation Syndrome (OHSS) (see WARNINGS-Overstimulation of the Ovary During Follistim® AQ Therapy) with or without pulmonary or vascular complications (see WARNINGS-Pulmonary and Vascular Complications) and multiple births (see WARNINGS-Multiple Births). Gonadotropin therapy requires the availability of appropriate monitoring facilities (see PRECAUTIONS-Laboratory Tests).
Overstimulation of the Ovary During Follistim® AQ Therapy
In order to minimize the hazards associated with the occasional abnormal ovarian enlargement that may occur with Follistim® AQ therapy, the lowest effective dose should be used (see DOSAGE AND ADMINISTRATION). Use of ultrasound monitoring of ovarian response and/or measurement of serum estradiol levels can further minimize the risk of overstimulation.
If the ovaries are abnormally enlarged on the last day of Follistim® AQ therapy, hCG should not be administered in this course of treatment; to reduce the chances of developing Ovarian Hyperstimulation Syndrome (OHSS).
The Ovarian Hyperstimulation Syndrome (OHSS): OHSS is a medical entity distinct from uncomplicated ovarian enlargement and may progress rapidly to become a serious medical event. OHSS is characterized by a dramatic increase in vascular permeability, which can result in a rapid accumulation of fluid in the peritoneal cavity, thorax, and potentially, the pericardium. The early warning signs of OHSS developing are severe pelvic pain, nausea, vomiting, and weight gain. The following symptoms have been reported in cases of OHSS: abdominal pain, abdominal distension, gastrointestinal symptoms including nausea, vomiting and diarrhea, severe ovarian enlargement, weight gain, dyspnea, and oliguria. Clinical evaluation may reveal hypovolemia, hemoconcentration, electrolyte imbalances, ascites, hemoperitoneum, pleural effusions, hydrothorax, acute pulmonary distress, and thromboembolic events (see WARNINGS-Pulmonary and Vascular Complications). Transient liver function test abnormalities suggestive of hepatic dysfunction, which may be accompanied by morphologic changes on liver biopsy, have been reported in association with Ovarian Hyperstimulation Syndrome (OHSS).
During clinical trials with Follistim® therapy, OHSS occurred in 53 (5.2%) of the 1029 women treated and of these 29 (2.8%) were hospitalized. Cases of OHSS are more common, more severe, and more protracted if pregnancy occurs; therefore, patients should be followed for at least two weeks after hCG administration. Most often, OHSS occurs after treatment has been discontinued and it can develop rapidly, reaching its maximum about seven to ten days following treatment. Usually, OHSS resolves spontaneously with the onset of menses. If there is evidence that OHSS may be developing prior to hCG administration (see PRECAUTIONS-Laboratory Tests), the hCG must be withheld.
If serious OHSS occurs, treatment should be stopped and the patient should be hospitalized. Treatment is primarily symptomatic and should consist of bed rest, fluid and electrolyte management, and analgesics (if needed). Hemoconcentration associated with fluid loss into the peritoneal cavity, pleural cavity, and the pericardial cavity may occur and should be thoroughly assessed in the following manner: 1) fluid intake and output; 2) weight; 3) hematocrit; 4) serum and urinary electrolytes; 5) urine specific gravity; 6) BUN and creatinine; 7) total proteins with albumin: globulin ratio; 8) coagulation studies; 9) electrocardiogram to monitor for hyperkalemia and 10) abdominal girth. These determinations should be performed daily or more often based on clinical need.
OHSS increases the risk of injury to the ovary. The ascitic, pleural, and pericardial fluid should not be removed unless there is the necessity to relieve symptoms such as pulmonary distress or cardiac tamponade. Pelvic examination may cause rupture of an ovarian cyst, which may result in hemoperitoneum, and should therefore be avoided. If bleeding occurs and requires surgical intervention, the clinical objective should be to control the bleeding and retain as much ovarian tissue as possible. Intercourse should be prohibited in patients with significant ovarian enlargement after ovulation because of the danger of hemoperitoneum resulting from ruptured ovarian cysts.
The management of OHSS may be divided into three phases: an acute, a chronic, and a resolution phase. Because the use of diuretics can accentuate the diminished intravascular volume, diuretics should be avoided except in the late phase of resolution as described below.
Acute Phase: Management during the acute phase should be directed at preventing hemoconcentration due to loss of intravascular volume to the third space and minimizing the risk of thromboembolic phenomena and kidney damage. Treatment is intended to normalize electrolytes while maintaining an acceptable but somewhat reduced intravascular volume. Full correction of the intravascular volume deficit may lead to an unacceptable increase in the amount of third space fluid accumulation.
Management includes administration of limited intravenous fluids, electrolytes, human serum albumin and strict monitoring of fluid intake and output. Monitoring for the development of hyperkalemia is recommended.
Chronic Phase: After stabilizing the patient during the acute phase, excessive fluid accumulation in the third space should be limited by instituting severe potassium, sodium, and fluid restriction.
Resolution Phase: A fall in hematocrit and an increasing urinary output without an increased intake are observed due to the return of the third space fluid to the intravascular compartment. Peripheral and/or pulmonary edema may result if the kidneys are unable to excrete third space fluid as rapidly as it is mobilized. Diuretics may be indicated during the resolution phase, if necessary, to combat pulmonary edema.
Pulmonary and Vascular Complications
Serious pulmonary conditions (e.g., atelectasis, acute respiratory distress syndrome) have been reported in women treated with gonadotropins. In addition, thromboembolic events both in association with, and separate from, the Ovarian Hyperstimulation Syndrome have been reported following gonadotropin therapy. Intravascular thrombosis, which may originate in venous or arterial vessels, can result in reduced blood flow to vital organs or the extremities. Sequelae of such events have included venous thrombophlebitis, pulmonary embolism, pulmonary infarction, cerebral vascular occlusion (stroke), and arterial occlusion resulting in loss of limb. In rare cases, pulmonary complications and/or thromboembolic events have resulted in death.
Multiple Births
Multiple births have been reported for all FSH treatments including Follistim® AQ (follitropin beta injection) treatment. The patient and her partner should be advised of the potential risk of multiple births before starting treatment.
PRECAUTIONS
General
Careful attention should be given to the diagnosis of infertility and in the selection of candidates for Follistim® AQ (follitropin beta injection) therapy (see INDICATIONS AND USAGE-Selection of Patients).
Information for Patients
Prior to therapy with Follistim® AQ, patients should be informed of the duration of treatment and monitoring procedures that will be required. The risks of Ovarian Hyperstimulation Syndrome and multiple births (see WARNINGS), and other possible adverse reactions (see ADVERSE REACTIONS) should be discussed.
Laboratory Tests
In most instances, treatment with Follistim® AQ will result only in follicular growth and maturation. In order to complete the final phase of follicular maturation and to induce ovulation, hCG must be given following the administration of Follistim® AQ or when clinical assessment of the patient indicates that sufficient follicular maturation has occurred. This may be directly estimated by sonographic visualization of the ovaries and endometrial lining and/or measuring serum estradiol levels. The combination of both ultrasonography and measurement of estradiol levels is useful for monitoring the growth and development of follicles, timing hCG administration, as well as minimizing the risk of OHSS and multiple gestations.
The clinical confirmation of ovulation is obtained by direct and indirect indices of progesterone production. The indices most generally used are as follows:
- A rise in basal body temperature,
- Increase in serum progesterone, and
- Menstruation following the shift in basal body temperature.
When used in conjunction with indices of progesterone production, sonographic visualization of the ovaries will assist in determining if ovulation has occurred. Sonographic evidence of ovulation may include the following:
- Fluid in the cul-de-sac,
- Follicle showing marked decrease in size, and
- Collapsed follicle.
Drug Interactions
No drug-drug interaction studies have been performed.
Carcinogenesis and Mutagenesis, Impairment of Fertility
Long-term toxicity studies in animals have not been performed with Follistim® to evaluate the carcinogenic potential of the drug. Follistim® was not mutagenic in the Ames test using S. typhimurium and E. coli tester strains and did not produce chromosomal aberrations in an in vitro assay using human lymphocytes.
Pregnancy
Pregnancy Category X: (See CONTRAINDICATIONS).
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in the nursing infant from Follistim® AQ, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of Follistim® did not include subjects aged 65 and over.
ADVERSE REACTIONS
Assisted Reproductive Technologies (ART)
Rates of adverse events from a randomized, assessor-blind, group comparative, multicenter safety and efficacy study of Follistim® in 989 infertile women treated with in vitro fertilization with Follistim® or urofollitropin after pituitary suppression with a GnRH agonist are summarized in Table 5.
| Adverse Event | Follistim® (n=591) |
| Miscarriage | 11.0% |
| Ovarian Hyperstimulation Syndrome | 5.2% |
| Ectopic pregnancy | 3.0% |
| Abdominal pain | 2.5% |
| Injection site pain | 1.7% |
| Vaginal hemorrhage | 1.5% |
Ovulation Induction
Rates of adverse events from a randomized, assessor-blind, group comparative, multicenter safety and efficacy study of Follistim® in 172 chronic anovulatory women who failed to ovulate and/or conceive during clomiphene citrate treatment are summarized in Table 6.
| Adverse Event | Follistim® (n=105) |
| Miscarriage | 9.5% |
| Ovarian Hyperstimulation Syndrome | 7.6% |
| Abdominal discomfort | 2.9% |
| Abdominal pain, lower | 2.9% |
| Abdominal pain | 1.9% |
| Ovarian cyst | 2.9% |
The following adverse events have been reported in women treated with gonadotropins: pulmonary and vascular complications (see WARNINGS), hemoperitoneum, adnexal torsion (as a complication of ovarian enlargement), dizziness, tachycardia, dyspnea, tachypnea, febrile reactions, flu-like symptoms including fever, chills, musculoskeletal aches, joint pains, nausea, headache and malaise, breast tenderness, and dermatological symptoms (dry skin, erythema, body rash, hair loss, and hives).
There have been infrequent reports of ovarian neoplasms, both benign and malignant, in women who have undergone multiple drug regimens for ovulation induction; however, a causal relationship has not been established.
Congenital Anomalies
The incidence of congenital malformations after Assisted Reproductive Technologies (ART) may be slightly higher than after spontaneous conception. This slightly higher incidence is thought to be related to differences in parental characteristics (e.g., maternal age, sperm characteristics) and to the higher incidence of multiple gestations after ART. There are no indications that the use of gonadotropins during ART is associated with an increased risk of congenital malformations.
DRUG ABUSE AND DEPENDENCE
There have been no reports of abuse or dependence with Follistim® AQ (follitropin beta injection).
OVERDOSAGE
Aside from the possibility of Ovarian Hyperstimulation Syndrome (see WARNINGS-Overstimulation of the Ovary During Follistim® AQ Therapy) and multiple gestations (see WARNINGS-Multiple Births), there is no additional information concerning the consequences of acute overdosage with Follistim® AQ (follitropin beta injection).
DOSAGE AND ADMINISTRATION
Assisted Reproductive Technologies (ART)
A starting dose of 150 to 225 IU of Follistim® AQ (follitropin beta injection) is recommended for at least the first four days of treatment. After this, the dose may be adjusted for the individual patient based upon their ovarian response. In clinical studies with patients who are responding, it was shown that daily maintenance dosages ranging from 75 to 300 IU for six to twelve days are sufficient, although longer treatment may be necessary. However, in patients that were low or poor responders, maintenance doses of 375 to 600 IU were administered according to individual response. This later category comprised approximately 10% of the women evaluated during clinical studies. The maximum, individualized, daily dose of Follistim® that has been used in clinical studies is 600 IU. When a sufficient number of follicles of adequate size are present, the final maturation of the follicles is induced by administering hCG at a dose of 5000 IU to 10,000 IU. Oocyte (egg) retrieval is performed 34 to 36 hours later. The administration of hCG must be withheld in cases where the ovaries are abnormally enlarged on the last day of Follistim® AQ therapy. This will reduce the chance of developing OHSS.
Ovulation Induction
In studies using Follistim®, a stepwise gradually increasing dosing scheme was used. The starting dose was 75 IU of Follistim® for up to 14 days. The dose was then increased by 37.5 IU of Follistim® at weekly intervals until follicular growth and/or serum estradiol levels indicated an adequate response. The maximum, individualized, daily dose of Follistim® that has been safely used for ovulation induction patients during clinical trials is 300 IU. Treatment should continue until ultrasonic visualizations and/or serum estradiol determinations indicate pre-ovulatory conditions equivalent to or greater than those of the normal individual followed by hCG, 5000 to 10,000 IU. If the ovaries are abnormally enlarged on the last day with Follistim® AQ therapy, hCG must be withheld during this course of treatment; this will reduce the chances of developing OHSS.
During treatment with Follistim® AQ and during a two-week post-treatment period, patients should be examined at least every other day for signs of excessive ovarian stimulation. It is recommended that Follistim® AQ administration be stopped if the ovaries become abnormally enlarged or abdominal pain occurs. Most OHSS occurs after treatment has been discontinued and reaches its maximum at about seven to ten days post-ovulation.
For ovulation induction, the couple should be encouraged to have intercourse daily, beginning on the day prior to the administration of hCG and until ovulation becomes apparent from the indices employed for the determination of progestational activity (see PRECAUTIONS-Laboratory Tests). Care should be taken to insure insemination. In the light of the foregoing indices and parameters mentioned, it should become obvious that, unless a physician is willing to devote considerable time to these patients and be familiar with and conduct these necessary laboratory studies, he/she should not use Follistim® AQ.
Directions for using Follistim® AQ
- Please read these instructions carefully before using Follistim® AQ.
- Before you begin, check the liquid in the container. If the solution is not clear and colorless or has particles in it, DO NOT USE. Please consult your healthcare provider.
- Injecting cold drug is likely to cause discomfort. Therefore, it is recommended you allow the drug to reach room temperature before taking the injection.
What you will need before giving yourself the injection
- Only use the syringe and needle(s) prescribed by your physician.
- Alcohol, cotton balls or alcohol pads/swabs, sterile gauze, antibacterial soap and a special safety container to discard the used needles, and/or other supplies.
- A clean dry surface for preparation of your injection.
Before using Follistim® AQ (follitropin beta injection) for the first time, read these instructions carefully. Keep this leaflet in a safe place and refer to it when questions arise.
- Wash your hands thoroughly with antibacterial soap and water before
starting.
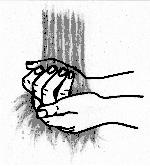
- Remove the flip-cap off of the vial and wipe the rubber stopper with an
alcohol swab.
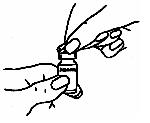
- Use a fresh alcohol swab to clean about two inches around the injection site
where the needle will be inserted, as explained by your healthcare provider (See
step 6 below).Let the alcohol dry on your skin for at least one minute before injecting the medicine.

-
- Attach a needle to the syringe. Carefully remove the cap from the needle
and pierce it through the rubber stopper of the vial.
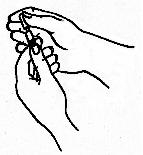
- Draw the volume of Follistim® AQ into the syringe as prescribed by your
healthcare provider.
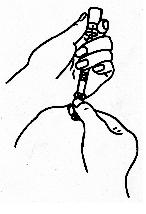
- Attach a needle to the syringe. Carefully remove the cap from the needle
and pierce it through the rubber stopper of the vial.
- Hold the syringe pointing upwards and gently tap the side to force any air
bubbles to the top; then squeeze the plunger gently until all the air has been
expelled and a drop of Follistim® AQ solution appears at the tip of the
needle.
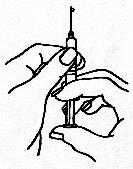
- Follistim® AQ is designed to be injected SUBCUTANEOUSLY or INTRAMUSCULARLY. Your healthcare provider will decide which type of
injection is best for you.
-
SUBCUTANEOUS INJECTION
- Carefully follow your healthcare provider’s instructions for
administering Follistim® AQ subcutaneously. The best place for SUBCUTANEOUS INJECTION is in the area just
below the belly button (navel) or in the upper thigh (upper
leg).
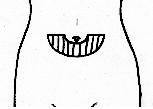
- Change your injection site a little with each injection to lower your chances for skin reactions.
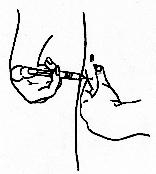
- For SUBCUTANEOUS injection,
pinch up a large area of skin between the finger and thumb. The needle
should be inserted at an angle of 90° to the skin
surface.
- Carefully follow your healthcare provider’s instructions for
administering Follistim® AQ subcutaneously. The best place for SUBCUTANEOUS INJECTION is in the area just
below the belly button (navel) or in the upper thigh (upper
leg).
-
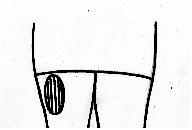
INTRAMUSCULAR INJECTION
- Carefully follow your healthcare provider’s instructions for
administering Follistim® AQ intramuscularly. You may consider asking
another person for assistance. The best site for INTRAMUSCULAR injection of Follistim® AQ is
the upper outer quadrant of the buttock muscle. Stretching the skin
helps the needle to go in more easily and pushes the tissue beneath the
skin out of the way. This helps the solution disperse
correctly.
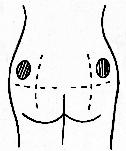
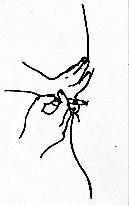
- Relax the muscle first by shifting your weight to the leg opposite
the muscle to be injected. The needle for INTRAMUSCULAR injection should be fully inserted to the hub
of the needle, at an angle of 90° to the skin surface.
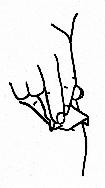
- Pull back gently on the plunger. If the needle is correctly positioned it will be difficult to draw the plunger back. Any blood drawn into the syringe means the needle tip has penetrated a blood vessel. If this happens, remove the syringe, cover the injection site with an alcohol swab and apply pressure. The site should stop bleeding in a minute or two. Discard original needle and replace with a new sterile needle. Repeat the above steps for intramuscular administration at a different site.
- Carefully follow your healthcare provider’s instructions for
administering Follistim® AQ intramuscularly. You may consider asking
another person for assistance. The best site for INTRAMUSCULAR injection of Follistim® AQ is
the upper outer quadrant of the buttock muscle. Stretching the skin
helps the needle to go in more easily and pushes the tissue beneath the
skin out of the way. This helps the solution disperse
correctly.
-
SUBCUTANEOUS INJECTION
- Once the needle is properly placed, depress the plunger slowly and steadily, so the solution is correctly injected.
- Pull the syringe out quickly and apply pressure to the site with an alcohol
swab. Gently massage the site—while still maintaining pressure—to help disperse
the Follistim® AQ solution and relieve any discomfort.
- Use the syringe, needle, and Follistim® AQ vial only once and dispose of them properly as instructed by your healthcare provider.
General information about Follistim® AQ
- If you miss or forget a dose, do not double your next dose. Contact your healthcare provider for recommendations.
- Do not mix Follistim® AQ with any other medicines in the same vial or in the same syringe.
HOW SUPPLIED
Follistim® AQ (follitropin beta injection) is supplied as a sterile aqueous solution in the following concentrations and packaging:
Follistim®
AQ Single-Use Vial 75 IU/0.5 mL
Box of
1
NDC 0052-0308-02
Follistim®
AQ Single-Use Vial 150 IU/0.5 mL
Box of
1
NDC 0052-0309-02
Storage
Store refrigerated, 2–8°C (36–46°F) until dispensed. Upon dispensing, the product may be stored by the patient at 2–8°C (36–46°F) until the expiration date, or at or below 25°C (77°F) for 3 months or until expiration date, whichever occurs first. Protect from light, keep container in carton. Do not freeze.
For more detail regarding
the information contained in this leaflet call
1-866-836-5633.
www.follistim.com
Follistim® is a registered trademark of
N.V. Organon.
Manufactured for Organon USA Inc.
Roseland, NJ 07068
by Organon
(Ireland) Ltd.
Swords, Co. Dublin, Ireland
©2005 Organon USA
Inc.
SUPPLEMENTAL PATIENT MATERIAL
Please note that the following instructions are for Follistim® AQ packaged in a single-use vial, and should not be confused with Follistim® AQ Cartridge for use with Follistim Pen®. If your physician prescribed Follistim® AQ Cartridge, please see the Patient Information Leaflet and INSTRUCTIONS FOR USE booklet accompanying that product before continuing.
Read the patient information carefully before you start using Follistim® AQ (follitropin beta injection). This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is Follistim® AQ?
Follistim® AQ (follitropin beta injection) is a medicine that contains the follicle-stimulating hormone (FSH). FSH helps (stimulate) healthy ovaries to make eggs.
What is Follistim® AQ used for?
Doctors specializing in treating women who have problems getting pregnant use Follistim® AQ (follitropin beta injection) for the patient who needs medical help to have a child because that patient:
- has problems with ovulation (producing and releasing eggs). Follistim® AQ will not help women whose ovaries do not work at all (primary ovarian failure).
- is participating in an Assisted Reproductive Technology (ART) program, such as in vitro fertilization, to help her ovaries make more eggs.
Follistim® AQ should be used only for women seeking to become pregnant. Follistim® AQ may be one of several drugs prescribed to you as part of your treatment program. Always follow your doctor’s dosing instructions when administering Follistim® AQ. Your doctor has individualized the dose to be administered based on your medical history. Do not change your dose unless instructed by your doctor.
Who should not take Follistim® AQ?
Do not use Follistim® AQ (follitropin beta injection) if you:
- are allergic to recombinant human FSH products (see the end of this leaflet for a list of all the ingredients in Follistim® AQ)
- have primary ovarian failure (your ovaries do not work at all)
- are pregnant, or think you might be pregnant
- have uncontrolled thyroid or adrenal gland problems
- have tumors in your ovaries, breasts, uterus, hypothalamus, or pituitary gland
- have heavy or irregular vaginal bleeding and the cause is not known
- have ovarian cysts or enlarged ovaries, not due to polycystic ovary syndrome (PCOS)
- are allergic to streptomycin or neomycin. Follistim® AQ may contain traces of these antibiotics and may cause allergic reactions.
Tell your healthcare provider if you are breast-feeding. It is not known if Follistim® AQ passes into your milk.
What are the possible side effects of Follistim® AQ?
Follistim® AQ (follitropin beta injection) and other FSH products may cause serious side effects including:
-
Ovarian Hyperstimulation Syndrome
(OHSS). OHSS is a serious medical problem that can happen when the
ovaries are overstimulated. In rare cases it has caused death. OHSS causes fluid
to build up suddenly in the stomach and chest areas.
Stop using Follistim® AQ and call your healthcare provider right away if you get any of the following symptoms:- severe pelvic pain (lower stomach area)
- nausea
- vomiting
- sudden weight gain
- reduced urine output
- Lung and blood vessel problems. Follistim® AQ and other FSH products may cause serious lung problems including fluid in the lungs, trouble breathing and worsening of asthma. Follistim® AQ and other FSH products may also cause blood clots in blood vessels. This can lead to blood vessel problems (thrombophlebitis), stroke, loss of limb, or a blood clot in the lung (pulmonary embolus).
- Multiple births. Follistim® AQ and other FSH products can cause multiple births. Your healthcare provider will discuss your chances of multiple births.
Other side effects with Follistim® AQ include stomach pain, gas, pelvic pain, nausea, breast pain, injection site problems, enlarged stomach area, back pain, constipation, headache and ovarian pain. If you get any side effects that concern you, call your healthcare provider.
These are not all the side effects of Follistim® AQ. Contact your doctor or other healthcare provider without delay if you are experiencing symptoms including significant abdominal pain, or if symptoms develop some days after the last injection has been given (see “Who should not take Follistim® AQ?”).
How should I use Follistim® AQ?
- Use Follistim® AQ (follitropin beta injection) as prescribed for you. Your healthcare provider will decide on the dose of Follistim® AQ that is best for you. This dose may be increased or decreased as your treatment goes on. This will depend on your type of treatment. Do not change the dose of Follistim® AQ unless your healthcare provider tells you to. It is very important that you follow your healthcare provider’s instructions exactly.
- Follistim® AQ is given by an injection under the skin (subcutaneous injection) or into a muscle (intramuscular injection).
- Your healthcare provider will teach you how to inject yourself. See the end of this leaflet for “INSTRUCTIONS FOR USE”. Do not inject Follistim® AQ at home until your healthcare provider has taught you the right way.
Close care by your healthcare provider is very important. Usually, ultrasound scans of the ovaries are regularly made. Blood or urine samples are regularly taken. The results of these tests allow your healthcare provider to choose the right dose of Follistim® AQ for you each day. This is very important. Too high a dose of FSH may lead to rare, but serious problems as discussed above. Regular checking of your response to FSH treatment helps your healthcare provider lower your chances of ovarian overstimulation.
- If you use too much Follistim® AQ, call your healthcare provider right away.
- Call your healthcare provider right away if you get strong abdominal pain. Also call your healthcare provider right away if this happens some days after the last injection has been given.
- Do not use Follistim® AQ for a condition for which it was not prescribed.
- Do not give Follistim® AQ to other people, even if they have the same symptoms that you have.
PLEASE READ THESE INSTRUCTIONS CAREFULLY BEFORE USING FOLLISTIM® AQ.
- Before you begin, check the liquid in the container. If the solution is not clear and colorless or has particles in it, DO NOT USE. Please consult your healthcare provider.
- Injecting cold drug is likely to cause discomfort. Therefore, it is recommended you allow the drug to reach room temperature before taking the injection.
WHAT YOU WILL NEED FOR GIVING YOURSELF THE INJECTION
- Only use the syringe and needle(s) prescribed by your physician.
- Alcohol, cotton balls or alcohol pads/swabs, sterile gauze, antibacterial soap and a special safety container to discard the used needles, and/or other supplies.
- A clean dry surface for preparation of your injection.
DIRECTIONS FOR USING FOLLISTIM® AQ
Important notice
- Please read these instructions carefully before using Follistim® AQ.
- Before you begin, check the liquid in the container. If the solution is not clear and colorless or has particles in it, DO NOT USE. Please consult your healthcare provider.
- Injecting cold drug is likely to cause discomfort. Therefore, it is recommended you allow the drug to reach room temperature before taking the injection.
What you will need before giving yourself the injection
- Only use the syringe and needle(s) prescribed by your physician.
- Alcohol, cotton balls or alcohol pads/swabs, sterile gauze, antibacterial soap and a special safety container to discard the used needles, and/or other supplies.
- A clean dry surface for preparation of your injection.
Before using Follistim® AQ (follitropin beta injection) for the first time, read these instructions carefully. Keep this leaflet in a safe place and refer to it when questions arise.
- Wash your hands thoroughly with antibacterial soap and water before
starting.

- Remove the flip-cap off of the vial and wipe the rubber stopper with an
alcohol swab.

- Use a fresh alcohol swab to clean about two inches around the injection site
where the needle will be inserted, as explained by your healthcare provider (See
step 6 below).Let the alcohol dry on your skin for at least one minute before injecting the medicine.

-
- Attach a needle to the syringe. Carefully remove the cap from the needle
and pierce it through the rubber stopper of the vial.

- Draw the volume of Follistim® AQ into the syringe as prescribed by your
healthcare provider.

- Attach a needle to the syringe. Carefully remove the cap from the needle
and pierce it through the rubber stopper of the vial.
- Hold the syringe pointing upwards and gently tap the side to force any air
bubbles to the top; then squeeze the plunger gently until all the air has been
expelled and a drop of Follistim® AQ solution appears at the tip of the
needle.

- Follistim® AQ is designed to be injected SUBCUTANEOUSLY or INTRAMUSCULARLY. Your healthcare provider will decide which type of
injection is best for you.
-
SUBCUTANEOUS INJECTION
- Carefully follow your healthcare provider’s instructions for
administering Follistim® AQ subcutaneously. The best place for SUBCUTANEOUS INJECTION is in the area just
below the belly button (navel) or in the upper thigh (upper
leg).


- Change your injection site a little with each injection to lower your chances for skin reactions.
- For SUBCUTANEOUS injection,
pinch up a large area of skin between the finger and thumb. The needle
should be inserted at an angle of 90° to the skin
surface.

- Carefully follow your healthcare provider’s instructions for
administering Follistim® AQ subcutaneously. The best place for SUBCUTANEOUS INJECTION is in the area just
below the belly button (navel) or in the upper thigh (upper
leg).
-
INTRAMUSCULAR INJECTION
- Carefully follow your healthcare provider’s instructions for
administering Follistim® AQ intramuscularly. You may consider asking
another person for assistance. The best site for INTRAMUSCULAR injection of Follistim® AQ is
the upper outer quadrant of the buttock muscle. Stretching the skin
helps the needle to go in more easily and pushes the tissue beneath the
skin out of the way. This helps the solution disperse
correctly.

- Relax the muscle first by shifting your weight to the leg opposite
the muscle to be injected. The needle for INTRAMUSCULAR injection should be fully inserted to the hub
of the needle, at an angle of 90° to the skin surface.

- Pull back gently on the plunger. If the needle is correctly positioned it will be difficult to draw the plunger back. Any blood drawn into the syringe means the needle tip has penetrated a blood vessel. If this happens, remove the syringe, cover the injection site with an alcohol swab and apply pressure. The site should stop bleeding in a minute or two. Discard original needle and replace with a new sterile needle. Repeat the above steps for intramuscular administration at a different site.
- Carefully follow your healthcare provider’s instructions for
administering Follistim® AQ intramuscularly. You may consider asking
another person for assistance. The best site for INTRAMUSCULAR injection of Follistim® AQ is
the upper outer quadrant of the buttock muscle. Stretching the skin
helps the needle to go in more easily and pushes the tissue beneath the
skin out of the way. This helps the solution disperse
correctly.
-
SUBCUTANEOUS INJECTION
- Once the needle is properly placed, depress the plunger slowly and steadily, so the solution is correctly injected.
- Pull the syringe out quickly and apply pressure to the site with an alcohol
swab. Gently massage the site—while still maintaining pressure—to help disperse
the Follistim® AQ solution and relieve any discomfort.

- Use the syringe, needle, and Follistim® AQ vial only once and dispose of them properly as instructed by your healthcare provider.
General information about Follistim® AQ
- If you miss or forget a dose, do not double your next dose. Contact your healthcare provider for recommendations.
- Do not mix Follistim® AQ with any other medicines in the same vial or in the same syringe.
Ingredients in Follistim® AQ
Follistim® AQ contains the active ingredient follitropin beta. Inactive ingredients include sucrose, sodium citrate, L-methionine, polysorbate 20, water for injection, hydrochloric acid, and/or sodium hydroxide.
Storing Follistim® AQ
Store refrigerated, 2–8°C (36–46°F) until dispensed. Upon dispensing, the product may be stored by the patient at 2–8°C (36–46°F) until the expiration date, or at or below 25°C (77°F) for 3 months or until expiration date, whichever occurs first. Protect from light, keep container in carton. Do not freeze.
This leaflet summarizes important information for patients about Follistim® AQ. If you would like more information, talk with your healthcare provider. Be sure to discuss any questions you may have before beginning treatment. For more detail on information contained in this leaflet call 1-866-836-5633 or log onto www.follistim.com.
Rx OnlyFollistim® is a registered trademark of N.V. Organon.
Manufactured for Organon USA Inc.
Roseland, NJ 07068
by Organon
(Ireland) Ltd.
Swords, Co. Dublin, Ireland
©2005 Organon USA Inc.
| FOLLISTIM AQ
follitropin beta injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| FOLLISTIM AQ
follitropin beta injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
Revised: 10/2008Organon USA, Inc.