IBU- ibuprofen tablet
Bryant Ranch Prepack
----------
IBU®Tablets
BOXED WARNING
Cardiovascular Risk
- NSAIDs may cause an increased risk of serious cardiovascularthrombotic events, myocardial infarction, and stroke,which can be fatal. This risk may increase with duration ofuse. Patients with cardiovascular disease or risk factors forcardiovascular disease may be at greater risk (See ). WARNINGS
- IBU tablets are contraindicated for treatment of peri-operativepain in the setting of coronary artery bypass graft (CABG)surgery (See ). WARNINGS
Gastrointestinal Risk
- NSAIDS cause an increased risk of serious gastrointestinaladverse events including bleeding, ulceration, and perforationof the stomach or intestines, which can be fatal. These eventscan occur at any time during use and without warning symptoms.Elderly patients are at greater risk for serious gastrointestinalevents. (See ). WARNINGS
DESCRIPTION
IBU tablets contain the active ingredient ibuprofen, which is (±) -2 - ( - isobutylphenyl) propionic acid. Ibuprofen is a white powde rwith a melting point of 74-77° C and is very slightly soluble in water(<1 mg/mL) and readily soluble in organic solvents such as ethanol and acetone. The structural formula is represented below: p
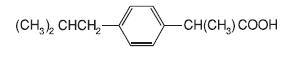
IBU, a nonsteroidal anti-inflammatory drug (NSAID), is availablein 400 mg, 600 mg, and 800 mg tablets for oral administration.Inactive ingredients: carnauba wax, colloidal silicon dioxide,croscarmellose sodium, hypromellose, magnesium stearate, microcrystallinecellulose, polydextrose, polyethylene glycol, polysorbate,titanium dioxide.
CLINICAL PHARMACOLOGY
IBU tablets contain ibuprofen which possesses analgesic andantipyretic activities. Its mode of action, like that of other NSAIDs, isnot completely understood, but may be related to prostaglandin synthetaseinhibition.
In clinical studies in patients with rheumatoid arthritis andosteoarthritis, Ibuprofen tablets have been shown to be comparableto aspirin in controlling pain and inflammation and to be associatedwith a statistically significant reduction in the milder gastrointestinalside effects (see ). Ibuprofen may be well toleratedin some patients who have had gastrointestinal side effectswith aspirin, but these patients when treated with IBU tablets shouldbe carefully followed for signs and symptoms of gastrointestinalulceration and bleeding. Although it is not definitely known whetheribuprofen causes less peptic ulceration than aspirin, in one studyinvolving 885 patients with rheumatoid arthritis treated for up to oneyear, there were no reports of gastric ulceration with ibuprofenwhereas frank ulceration was reported in 13 patients in the aspiringroup (statistically significant p<.001). ADVERSE REACTIONS
Gastroscopic studies at varying doses show an increased tendencytoward gastric irritation at higher doses. However, at comparabledoses, gastric irritation is approximately half that seen with aspirin.Studies using 51Cr-tagged red cells indicate that fecal blood lossassociated with Ibuprofen tablets in doses up to 2400 mg daily didnot exceed the normal range, and was significantly less than thatseen in aspirin-treated patients.
In clinical studies in patients with rheumatoid arthritis, Ibuprofenhas been shown to be comparable to indomethacin in controlling thesigns and symptoms of disease activity and to be associated with astatistically significant reduction of the milder gastrointestinal (see ) and CNS side effects. ADVERSE REACTIONS
Ibuprofen may be used in combination with gold salts and/or corticosteroids.
Controlled studies have demonstrated that Ibuprofen is a more effective analgesic than propoxyphene for the relief of episiotomy pain, pain following dental extraction procedures, and for the relief ofthe symptoms of primary dysmenorrhea.
In patients with primary dysmenorrhea, Ibuprofen has been shown to reduce elevated levels of prostaglandin activity in the menstrualfluid and to reduce resting and active intrauterine pressure, as well asthe frequency of uterine contractions. The probable mechanism ofaction is to inhibit prostaglandin synthesis rather than simply to provide analgesia.
The ibuprofen in IBU tablets is rapidly absorbed. Peak serum ibuprofen levels are generally attained one to two hours after administration.With single doses up to 800 mg, a linear relationship exists between amount of drug administered and the integrated area underthe serum drug concentration vs time curve. Above 800 mg, however,the area under the curve increases less than proportional to increases in dose. There is no evidence of drug accumulation or enzyme induction.
The administration of Ibuprofen tablets either under fasting conditions or immediately before meals yields quite similar serum ibuprofen concentration-time profiles. When Ibuprofen is administered immediately after a meal, there is a reduction in the rate of absorption but no appreciable decrease in the extent of absorption.The bioavailability of the drug is minimally altered by the presence of food.
A bioavailability study has shown that there was no interference with the absorption of ibuprofen when given in conjunction with anantacid containing both aluminum hydroxide and magnesium hydroxide.
Ibuprofen is rapidly metabolized and eliminated in the urine. The excretion of ibuprofen is virtually complete 24 hours after the last dose. The serum half-life is 1.8 to 2.0 hours.
Studies have shown that following ingestion of the drug, 45% to79% of the dose was recovered in the urine within 24 hours as metabolite A (25%), (+)-2-[ -(2hydroxymethyl-propyl) phenyl] propionic acid and metabolite B (37%), (+)-2-[ -(2carboxypropyl)phenyl]propionic acid; the percentages of free and conjugated ibuprofen were approximately 1% and 14%, respectively. pp
INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of Ibuprofentablets and other treatment options before deciding to use Ibuprofen.Use the lowest effective dose for the shortest duration consistent withindividual patient treatment goals (see ). WARNINGS
IBU tablets are indicated for relief of the signs and symptoms of rheumatoid arthritis and osteoarthritis.
IBU tablets are indicated for relief of mild to moderate pain.
IBU tablets are also indicated for the treatment of primary dysmenorrhea.
Controlled clinical trials to establish the safety and effectiveness of IBU tablets in children have not been conducted.
CONTRAINDICATIONS
IBU tablets are contraindicated in patients with known hypersensitivityto ibuprofen.
IBU tablets should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin orother NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS, Anaphylactoid Reactions,andPRECAUTIONS, Preexisting Asthma).
IBU tablets are contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery(see ). WARNINGS
PRECAUTIONS
General
IBU tablets cannot be expected to substitute for corticosteroids orto treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroidsmay lead to disease exacerbation. Patients on prolongedcorticosteroid therapy should have their therapy tapered slowly if adecision is made to discontinue corticosteroids.
The pharmacological activity of IBU tablets in reducing fever andinflammation may diminish the utility of these diagnostic signs indetecting complications of presumed noninfectious, painful conditions.
. Ophthalmological effects
Blurred and/or diminished vision, scotomata, and/or changes incolor vision have been reported. If a patient develops such complaintswhile receiving IBU tablets, the drug should be discontinued, and thepatient should have an ophthalmologic examination which includescentral visual fields and color vision testing.
Information for Patients
Patients should be informed of the following information beforeinitiating therapy with an NSAID and periodically during the course ofongoing therapy. Patients should also be encouraged to read theNSAID Medication Guide that accompanies each prescription dispensed
• IBU tablets like other NSAIDs, may cause serious CV side effects,such as MI or stroke, which may result in hospitalization and evendeath. Although serious CV events can occur without warningsymptoms, patients should be alert for the signs and symptoms ofchest pain, shortness of breath, weakness, slurring of speech, andshould ask for medical advice when observing any indicative sign orsymptoms. Patients should be apprised of the importance of thisfollow-up (see ). WARNINGS,Cardiovascular Effects
• IBU tablets, like other NSAIDs, can cause GI discomfort and, rarely,serious GI side effects, such as ulcers and bleeding, which mayresult in hospitalization and even death. Although serious GI tractulcerations and bleeding can occur without warning symptoms,patients should be alert for the signs and symptoms of ulcerationsand bleeding, and should ask for medical advice when observingany indicative signs or symptoms including epigastric pain, dyspepsia,melena, and hematemesis. Patients should be apprised of theimportance of this follow-up (see WARNINGS,Gastrointestinal Effects-Risk of Ulceration,Bleeding and Perforation).
• IBU tablets, like other NSAIDs, can cause serious skin side effectssuch as exfoliative dermatitis, SJS and TEN, which may result inhospitalization and even death. Although serious skin reactions mayoccur without warning, patients should be alert for the signs andsymptoms of skin rash and blisters, fever, or other signs of hypersensitivitysuch as itching, and should ask for medical advice whenobserving any indicative sign or symptoms. Patients should beadvised to stop the drug immediately if they develop any type ofrash and contact their physicians as soon as possible.
• Patients should promptly report signs or symptoms of unexplainedweight gain or edema to their physicians.
• Patients should be informed of the warning signs and symptoms ofhepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice,right upper quadrant tenderness and “flu-like” symptoms). If theseoccur, patients should be instructed to stop therapy and seek immediatemedical therapy.
• Patients should be informed of the signs of an anaphylactoid reaction(e.g. difficulty breathing, swelling of the face or throat). If theseoccur, patients should be instructed to seek immediate emergencyhelp (see . WARNINGS)
• In late pregnancy, as with other NSAIDs, IBU tablets should beavoided because it may cause premature closure of the ductus arteriosus.
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur withoutwarning symptoms, physicians should monitor for signs orsymptoms of GI bleeding. Patients on long-term treatment withNSAIDs should have their CBC and chemistry profile checked periodically.If clinical signs and symptoms consistent with liver or renaldisease develop, systemic manifestations occur (e.g., eosinophilia,rash etc.), or abnormal liver tests persist or worsen, IBU tabletsshould be discontinued.
Drug Interactions
Reports suggest that NSAIDs may diminish the antihypertensiveeffect of ACE-inhibitors. This interaction should be given considerationin patients taking NSAIDs concomitantly with ACE-inhibitors. ACE-inhibitors:
When IBU tablets are administered with aspirin, its protein bindingis reduced, although the clearance of free IBU tablets is notaltered. The clinical significance of this interaction is not known; however,as with other NSAIDs, concomitant administration of ibuprofenand aspirin is not generally recommended because of the potential forincreased adverse effects. Aspirin
Pregnancy
Teratogenic effects: Pregnancy Category C
Reproductive studies conducted in rats and rabbits have notdemonstrated evidence of developmental abnormalities. However,animal reproduction studies are not always predictive of humanresponse. There are no adequate and well-controlled studies in pregnantwomen. Ibuprofen should be used in pregnancy only if thepotential benefit justifies the potential risk to the fetus.
ADVERSE REACTIONS
The most frequent type of adverse reaction occurring withIbuprofen tablets is gastrointestinal. In controlled clinical trials thepercentage of patients reporting one or more gastrointestinal complaintsranged from 4% to 16%.
In controlled studies when Ibuprofen tablets were compared toaspirin and indomethacin in equally effective doses, the overall incidenceof gastrointestinal complaints was about half that seen in eitherthe aspirin- or indomethacin-treated patients.
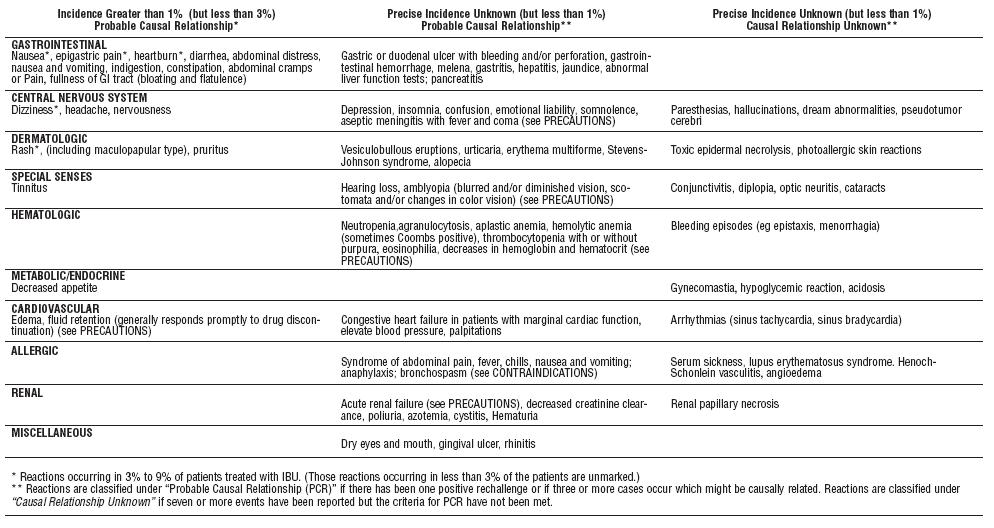
Adverse reactions observed during controlled clinical trials at anincidence greater than 1% are listed in the table. Those reactions listedin Column one encompass observations in approximately 3,000patients. More than 500 of these patients were treated for periods ofat least 54 weeks.
Still other reactions occurring less frequently than 1 in 100 werereported in controlled clinical trials and from marketing experience.These reactions have been divided into two categories: Column twoof the table lists reactions with therapy with Ibuprofen tablets wherethe probability of a causal relationship exists: for the reactions inColumn three, a causal relationship with Ibuprofen tablets has notbeen established.
Reported side effects were higher at doses of 3200 mg/day thanat doses of 2400 mg or less per day in clinical trials of patients withrheumatoid arthritis. The increases in incidence were slight and stillwithin the ranges reported in the table.
OVERDOSAGE
Approximately 11⁄2 hours after the reported ingestion of from 7 to10 Ibuprofen tablets (400 mg), a 19-month old child weighing 12 kgwas seen in the hospital emergency room, apneic and cyanotic,responding only to painful stimuli. This type of stimulus, however,was sufficient to induce respiration. Oxygen and parenteral fluidswere given; a greenish-yellow fluid was aspirated from the stomachwith no evidence to indicate the presence of ibuprofen. Two hoursafter ingestion the child’s condition seemed stable; she still respondedonly to painful stimuli and continued to have periods of apnea lastingfrom 5 to 10 seconds. She was admitted to intensive care andsodium bicarbonate was administered as well as infusions of dextroseand normal saline. By four hours post-ingestion she could bearoused easily, sit by herself and respond to spoken commands.Blood level of ibuprofen was 102.9 μg/mL approximately 81⁄2 hoursafter accidental ingestion. At 12 hours she appeared to be completelyrecovered.
In two other reported cases where children (each weighingapproximately 10 kg) accidentally, acutely ingested approximately120 mg/kg, there were no signs of acute intoxication or late sequelae.Blood level in one child 90 minutes after ingestion was 700 μg/mL —about 10 times the peak levels seen in absorption-excretion studies.A 19-year old male who had taken 8,000 mg of ibuprofen over aperiod of a few hours complained of dizziness, and nystagmus wasnoted. After hospitalization, parenteral hydration and three days bedrest, he recovered with no reported sequelae.
In cases of acute overdosage, the stomach should be emptied byvomiting or lavage, though little drug will likely be recovered if morethan an hour has elapsed since ingestion. Because the drug is acidicand is excreted in the urine, it is theoretically beneficial to administeralkali and induce diuresis. In addition to supportive measures, the useof oral activated charcoal may help to reduce the absorption andreabsorption of Ibuprofen tablets.
DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of IBU tabletsand other treatment options before deciding to use IBU tablets. Usethe lowest effective dose for the shortest duration consistent withindividual patient treatment goals (see WARNINGS).
After observing the response to initial therapy with IBU tablets, thedose and frequency should be adjusted to suit an individual patient’sneeds.Do not exceed 3200 mg total daily dose. If gastrointestinal complaintsoccur, administer IBU tablets with meals or milk.
| IBU
ibuprofen tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-1468), RELABEL(63629-1468) | |
