ANTIBIOTIC
-
neomycin sulfate ointment
Morales Distributors Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
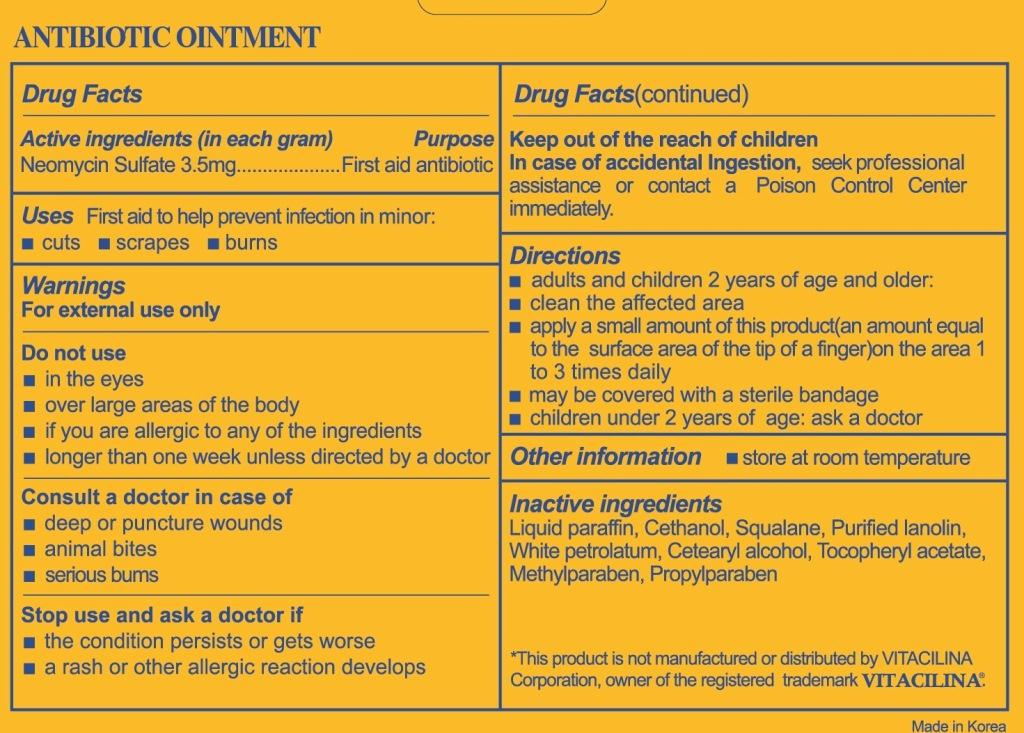
Do not use
- in the eyes
- over large areas of the body
- if you are allergic to any of the ingredients
- longer than one week unless directed by a doctor
Stop use and ask a doctor if
- the condition persists or gets worse
- a rash or other allergic reaction develops
Keep out of reach of children
in case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
- adults and children 2 years of age and older
- clean the affected area
- apply a small amount of this product(an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: ask a doctor
Inactive ingredients
Liquid paraffin, Cethanol, Squalane, Purified lanolin, White petrolatum, Cetearyl alcohol, Tocopheryl acetate, Methylparaben, Propylparaben
Package label
Compare to Vitacilinia First Aid Antibiotic active ingredient
MAXIMUM STRENGTH
ANTIBIOTIC OINTMENT
Neomycin Sulfate
FIRST AID ANTIBIOTIC OINTMENT
Net 1 oz (28.3g)
Exclusively distributed by:
Morales Distributors, Inc.
Mayaguez, P.R. 00682
Made in Korea

| ANTIBIOTIC
neomycin sulfate ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part333B | 09/25/2012 | |
| Labeler - Morales Distributors Inc (127151731) |
Revised: 09/2012 Morales Distributors Inc