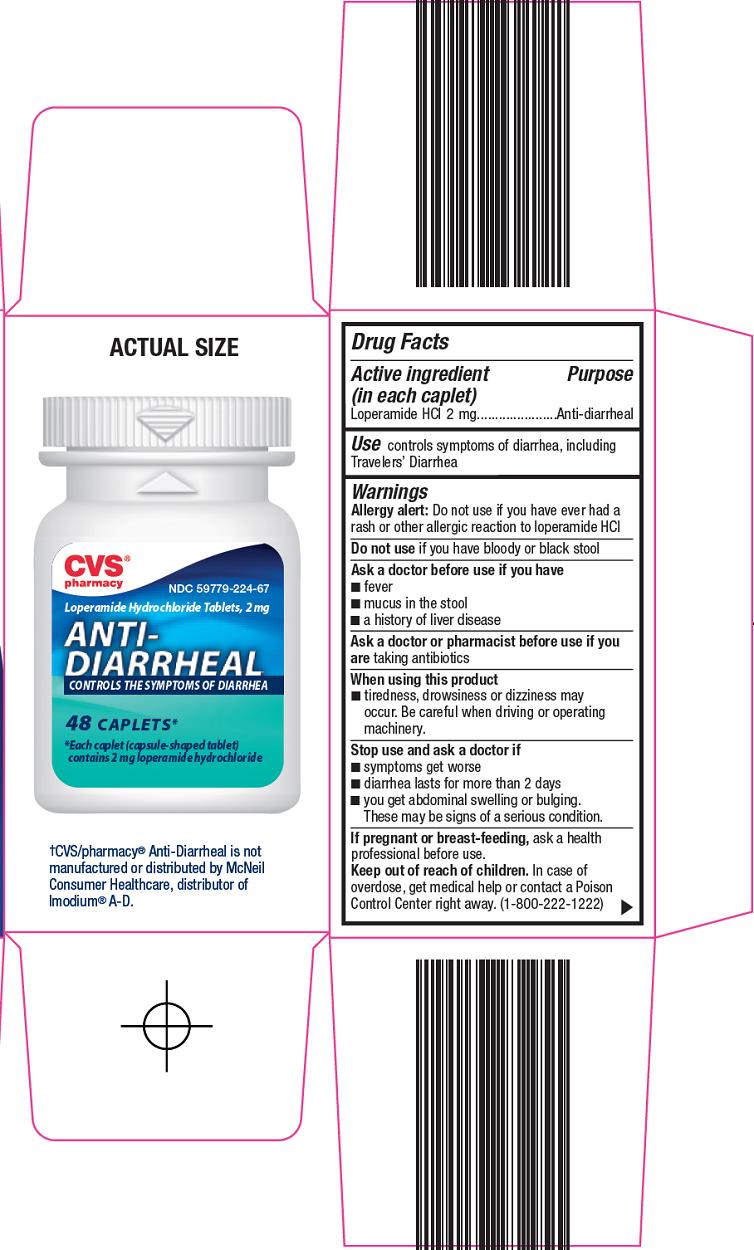
ANTI DIARRHEAL
-
loperamide hydrochloride tablet, film coated
CVS Pharmacy
----------
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl
When using this product
- •
- tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
Directions
- •
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- •
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
|
adults and children 12 years and over |
2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
|
children 9-11 years (60-95 lbs) |
1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
|
children 6-8 years (48-59 lbs) |
1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
|
children under 6 years (up to 47 lbs) |
ask a doctor |
Other information
- •
- store at 20°-25°C (68°-77°F)
- •
- do not use if printed foil under cap is broken or missing
- •
- see end panel for lot number and expiration date
Inactive ingredients
anhydrous lactose, carnauba wax, D&C yellow no. 10 aluminum lake, FD&C blue no. 1 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch
Principal Display Panel
Compare to the active ingredient in Imodium® A-D
Loperamide Hydrochloride Tablets, 2 mg
ANTI-DIARRHEAL
CONTROLS THE SYMPTOMS OF DIARRHEA
Actual Size
*Each caplet (capsule-shaped tablet) contains 2 mg loperamide hydrochloride
Easy to swallow
| ANTI DIARRHEAL
loperamide hydrochloride tablet, film coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA075232 | 02/24/2003 | |
| Labeler - CVS Pharmacy (062312574) |
Revised: 09/2012 CVS Pharmacy