CAVAN HEME OB
-
folic acid,
cholecalciferol,
.alpha.-tocopherol succinate, d-,
thiamine mononitrate,
riboflavin,
niacin,
pyridoxine hydrochloride,
cyanocobalamin,
biotin,
calcium pantothenate ,
iron,
potassium iodide,
zinc oxide,
sodium selenate and
cupric sulfate tablet
Seton Pharmaceuticals
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
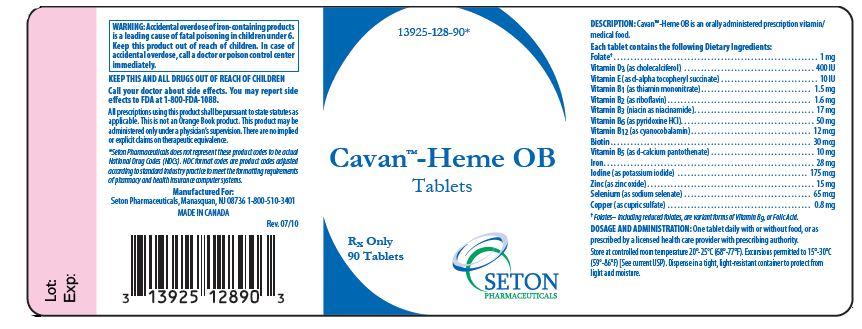
DESCRIPTION
Cavan™-Heme OB is an orally administered prescription vitamin/medical food.
| Each tablet contains the following Dietary Ingredients: | |
|---|---|
| Folate1 | 1 mg |
| Vitamin D3 (as cholecalciferol) | 400 IU |
| Vitamin E (as d-alpha tocopheryl succinate) | 10 IU |
| Vitamin B1 (as thiamine mononitrate) | 1.5 mg |
| Vitamin B2 (as riboflavin) | 1.6 mg |
| Vitamin B3 (niacin as niacinamide) | 17 mg |
| Vitamin B6 (as pyridoxine HCI) | 50 mg |
| Vitamin B12 (as cyanocobalamin) | 12 mcg |
| Biotin | 30 mcg |
| Vitamin B5 (as d-calcium pantothenate) | 10 mg |
| Iron | 28 mg |
| Iodine (as potassium iodide) | 175 mcg |
| Zinc (as zinc oxide) | 15 mg |
| Selenium (as sodium selenate) | 65 mcg |
| Copper (as cupric sulfate) | 0.8 mg |
Ingredients:
Folate1, Vitamin D3 (as cholecalciferol), Vitamin E (as d-alpha tocopheryl succinate), Vitamin B1 (as thiamin mononitrate), Vitamin B2 (as riboflavin), Vitamin B3 (niacin as niacinamide), Vitamin B6 (as pyridoxine HCI), Vitamin B12 (as cyanocobalamin), Biotin, Vitamin B5 (as d-calcium pantothenate), Iron, Iodine (as potassium iodide), Zinc (as zinc oxide), Selenium (as sodium selenate), Copper (as cupric sulfate), Gum Arabic, PVP K30, Microcrystalline Cellulose, Citric Acid, TriPotassium Citrate, Croscarmellose Sodium, Fumed Silica, Magnesium Stearate, Stearic Acid, Hypromellose, Polyethylene Glycol, Titanium Dioxide, Talc, Black Iron Oxide, Carmine, Vegetable Oil, and Polysorbate 80.
- 1
- Folates– including reduced folates, are variant forms of Vitamin B9, or Folic Acid.
INDICATIONS AND USAGE
Cavan™-Heme OB is an orally administered prescription vitamin/medical food indicated for the supplemental requirements of patients with nutritional deficiencies or are in need of dietary supplementation. Cavan™-Heme OB should always be used under medical supervision.
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
General:
Folates (including folic acid and reduced folates), when administered as a single agent in doses above 0.1 mg daily, may obscure the detection of B12 deficiency (specifically, the administration of folic acid may reverse the hematological manifestations of B12 deficiency, including pernicious anemia, while not addressing the neurological manifestations). Reduced folates may be less likely than folic acid to mask vitamin B12 deficiency. Folate therapy alone is inadequate for the treatment of B12 deficiency.
PATIENT INFORMATION
Cavan™-Heme OB is a prescription vitamin/medical food for use only under medical supervision and direction.
INTERACTIONS
Drugs which may interact with folate include:
- First generation anticonvulsants: High dose folic acid may result in decreased serum levels for first generation anticonvulsants (carbamazepine, fosphenytoin, phenytoin, phenobarbital, primidone, valproic acid, valproate). This may possibly reduce the effectiveness of first generation anticonvulsants and/or increase the frequency of seizures in susceptible patients. Nevertheless, caution should be used when prescribing folates among patients who are receiving treatment with first generation anticonvulsants.
- Second-generation anticonvulsants: Information on second-generation anticonvulsants' (including, but not limited to, lamotrigine) effect on folate levels is limited and cannot be ruled out.
- Capecitabine: Folinic acid (5-formyltetrahydrofolate) may increase the toxicity of Capecitabine.
- Cholestyramine: Reduces folic acid absorption and reduces serum folate levels.
- Colestipol: Reduces folic acid absorption and reduces serum folate levels.
- Colchicine: Colchicine may decrease folate plasma levels.
- L-dopa: L-dopa may decrease folate plasma levels.
- Cycloserine: Reduces folic acid absorption and reduces serum folate levels.
- Dihydrofolate Reductase Inhibitors (DHFRI): DHFRIs block the conversion of folic acid to its active forms, and lower plasma and red blood cell folate levels. DHFRIs include aminopterin, methotrexate, pyrimethamine, triamterene, and trimethoprim. Caution should be exercised when using folate with folate antagonists. Patients, typically, should not be given folate simultaneously with a folate antagonist, for the purpose of reducing or preventing clinical toxicity, as the therapeutic effect of the antagonist may be nullified.
- Fluoxetine: Fluoxetine exerts a noncompetitive inhibition of the 5-methyltetrahydrofolate active transport in the intestine.
- Isotretinoin: Reduced folate levels have occurred in some patients taking isotretinoin.
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs): NSAIDs have been shown to inhibit some folate dependent enzymes in laboratory experiments. NSAIDs include ibuprofen, naproxen, indomethacin and sulindac.
- Oral Contraceptives: Serum folate levels may be depressed by oral contraceptive therapy.
- Methylprednisolone: Reduced serum folate levels have been noted after treatment with methylprednisolone.
- Pancreatic Enzymes, including, but not limited to pancreatin and pancrelipase: Reduced folate levels have occurred in some patients taking pancreatic extracts.
- Pentamidine: Reduced folate levels have been seen with prolonged intravenous pentamidine.
- Smoking and Alcohol: Reduced serum folate levels have been noted.
- Sulfasalazine: Inhibits the absorption and metabolism of folic acid.
- Metformin treatment in patients with type 2 diabetes decreases serum folate.
- Warfarin can produce significant impairment in folate status after a 6-month therapy.
- Folate may enhance the toxicity of fluorouracil.
- Concurrent administration of chloramphenicol and folate in folate-deficient patients may result in antagonism of the haematopoietic response to folate.
- Caution should be exercised with the concomitant use of folate and trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection as it is associated with increased rates of treatment failure and mortality in a placebo controlled study.
Drugs which may interact with pyridoxine hydrochloride:
Pyridoxine hydrochloride should not be given to patients receiving the drug levodopa, because the action of levodopa is antagonized by pyridoxine hydrochloride. However, pyridoxine hydrochloride may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid, as well as possibly the use of other forms of folates – including reduced folates. Paresthesia, somnolence, nausea and headaches have been reported with pyridoxine hydrochloride. Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body have been associated with cyanocobalamin.
DOSAGE AND ADMINISTRATION
One tablet daily with or without food, or as prescribed by a licensed health care provider with prescribing authority.
HOW SUPPLIED
Cavan™-Heme OB is supplied as purple, oval tablets with “S128” debossed on one side, dispensed in bottles of 90 tablets.
Product Code: 13925-128-902
- 2
- Seton Pharmaceuticals does not represent these product codes to be actual National Drug Codes (NDCs). NDC format codes are product codes adjusted according to standard industry practice to meet the formatting requirements of pharmacy and health insurance computer systems.
STORAGE
Store at controlled room temperature 20°-25°C (68°-77°F). Excursions permitted to 15°-30°C (59°-86°F) [See current USP]. Dispense in a tight, light-resistant container to protect from light and moisture.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Rx Only
Reserved for Professional Recommendation
Call your doctor about side effects. You may report side effects to FDA at 1-800-FDA-1088.
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.
Manufactured For:
Seton Pharmaceuticals,
Manasquan, NJ 08736
1-800-510-3401
MADE IN CANADA
Rev.7/10
SETON
PHARMACEUTICALS
PRINCIPAL DISPLAY PANEL:
NDC 13925-128-90
Cavan TM- Heme OB
Tablets
Rx Only
90 Tablets
SETON
PHARMACEUTICALS
| CAVAN HEME OB
folic acid, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine mononitrate, riboflavin, niacin, pyridoxine hydrochloride, cyanocobalamin, biotin, calcium pantothenate, iron, potassium iodide, zinc oxide, sodium selenate and cupric sulfate tablet |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 08/03/2010 | 06/10/2012 | |
| Labeler - Seton Pharmaceuticals (828898002) |
| Registrant - Seton Pharmaceuticals (828898002) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| VIVA | 253288898 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Rainbow Gold | 800695152 | REPACK | |
Revised: 08/2012 Seton Pharmaceuticals