ISOPTO CARBACHOL
-
carbachol solution
Alcon Laboratories, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION SECTION
ISOPTO® Carbachol (carbachol ophthalmic solution) is a cholinergic prepared as a sterile topical ophthalmic solution. The active ingredient is represented by the chemical structure:
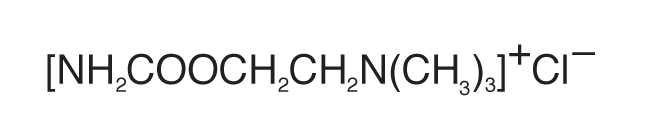
Established name: Carbachol
Chemical name: 2-[(Aminocarbonyl)oxy]-N,N,Ntrimethylethanaminium chloride.
Each mL contains: Active: carbachol, 1.5% or 3.0%. Preservative: benzalkonium chloride 0.005%. Vehicle: hypromellose 1.0%. Inactives: boric acid, sodium chloride, sodium borate, purified water.
CLINICAL PHARMACOLOGY SECTION
A cholinergic (parasympathomimetic) agent. Carbachol acts directly to stimulate muscarinic (smooth muscle) and nicotinic (autonomic ganglia) receptors and indirectly to inhibit cholinesterase enzyme activity. Carbachol is resistant to hydrolysis by cholinesterases.
CONTRAINDICATIONS SECTION
Miotics are contraindicated where pupillary constriction is undesirable such as acute iritis. Contraindicated in those persons showing hypersensitivity to any component of this preparation.
WARNINGS SECTION
FOR TOPICAL OPHTHALMIC USE ONLY. NOT FOR INJECTION. Carbachol should be used with caution in the presence of corneal abrasion to avoid excessive penetration which can produce systemic toxicity; and in patients with acute cardiac failure, bronchial asthma, active peptic ulcer, hyperthyroidism, gastrointestinal spasm, urinary tract obstruction, Parkinson’s disease, recent myocardial infarct, systemic hypertension or hypotension. As with all miotics, retinal detachment has been reported when used in certain susceptible individuals. Remove contact lenses before using.
PRECAUTIONS SECTION
Information for Patients
The miosis usually causes difficulty in dark adaptation. Patient should be advised to exercise caution in night driving and other hazardous occupations in poor light. Do not touch dropper tip to any surface, as this may contaminate the solution.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been conducted to evaluate the carcinogenic potential of carbachol.
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with carbachol. It is also not known whether carbachol can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Carbachol should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS SECTION
Transient symptoms of stinging and burning may occur. This preparation is capable of producing systemic symptoms of a cholinesterase inhibitor, even when the epithelium is intact. Transient ciliary and conjunctival injection, headache, and ciliary spasm with resultant temporary decrease of visual acuity may occur. Salivation, syncope, cardiac arrhythmia, gastrointestinal cramping, vomiting, asthma, hypotension, diarrhea, frequent urge to urinate, increased sweating, and irritation of eyes may occur.
OVERDOSAGE SECTION
Atropine should be administered parenterally (for dosage refer to Goodman & Gilman or other pharmacology reference).
DOSAGE & ADMINISTRATION SECTION
Instill two drops topically in the eye(s) up to three times daily or as indicated by physician.
HOW SUPPLIED SECTION
15 mL in plastic DROP-TAINER® Dispensers.
1.5%: 15 mL NDC 0998-0223-15
3%: 15 mL NDC 0998-0225-15
STORAGE SECTION
Store at 8° - 27°C (46° - 80°F).
Rx Only
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Revised: June 2007
Printed in USA
©2002, 2003, 2007 Alcon, Inc.
9002666-0607
PRINCIPAL DISPLAY PANEL
NDC 0998-0223-15
Alcon®
Isopto® Carbachol 1.5%
(carbachol ophthalmic solution)
15 mL Sterile


| ISOPTO CARBACHOL
carbachol solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 06/19/1981 | ||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Registrant - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Alcon Laboratories, Inc. | 008018525 | MANUFACTURE | |
Revised: 06/2007 Alcon Laboratories, Inc.