azmacort (Triamcinolone acetonide) aerosol, metered
[Abbott Laboratories]
Rx Only
For Oral Inhalation Only
Shake Well Before
Using
DESCRIPTION
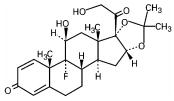
Triamcinolone acetonide, USP, the active ingredient in Azmacort® Inhalation Aerosol, is a corticosteroid with a molecular weight of 434.5 and with the chemical designation 9-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone. (C24H31FO6).
Azmacort Inhalation Aerosol is a metered-dose aerosol unit containing a microcrystalline suspension of triamcinolone acetonide in the propellant dichlorodifluoromethane and dehydrated alcohol USP 1% w/w. Each canister contains 60 mg triamcinolone acetonide. The canister must be primed prior to the first use. After an initial priming of 2 actuations, each actuation delivers 200 mcg triamcinolone acetonide from the valve and 75 mcg from the spacer-mouthpiece under defined in vitro test conditions. The canister will remain primed for 3 days. If the canister is not used for more than 3 days, then it should be reprimed with 2 actuations. There are at least 240 actuations in one Azmacort Inhalation Aerosol canister. After 240 actuations, the amount delivered per actuation may not be consistent and the unit should be discarded.
CLINICAL PHARMACOLOGY
Triamcinolone acetonide is a more potent derivative of triamcinolone. Although triamcinolone itself is approximately one to two times as potent as prednisone in animal models of inflammation, triamcinolone acetonide is approximately 8 times more potent than prednisone.
The precise mechanism of the action of glucocorticoids in asthma is unknown. However, the inhaled route makes it possible to provide effective local anti-inflammatory activity with reduced systemic corticosteroid effects. Though highly effective for asthma, glucocorticoids do not affect asthma symptoms immediately. While improvement in asthma may occur as soon as one week after initiation of Azmacort Inhalation Aerosol therapy, maximum improvement may not be achieved for 2 weeks or longer.
Based upon intravenous dosing of triamcinolone acetonide phosphate ester, the half-life of triamcinolone acetonide was reported to be 88 minutes. The volume of distribution (Vd) reported was 99.5 L (SD± 27.5) and clearance was 45.2 L/hour (SD ± 9.1) for triamcinolone acetonide. The plasma half-life of glucocorticoids does not correlate well with the biologic half-life.
The pharmacokinetics of radiolabeled triamcinolone acetonide [14C] were evaluated following a single oral dose of 800 mcg to healthy male volunteers. Radiolabeled triamcinolone acetonide was found to undergo relatively rapid absorption following oral administration with maximum plasma triamcinolone acetonide and [14C]-derived radioactivity occurring between 1.5 and 2 hours. Plasma protein binding of triamcinolone acetonide appears to be relatively low and consistent over a wide plasma triamcinolone acetonide concentration range as a function of time. The overall mean percent fraction bound was approximately 68%.
The metabolism and excretion of triamcinolone acetonide were both rapid and extensive with no parent compound being detected in the plasma after 24 hours post-dose and a low ratio (10.6%) of parent compound AUC0-∞ to total [14C] radioactivity AUC0-∞. Greater than 90% of the oral [14C]-radioactive dose was recovered within 5 days after administration in 5 out of the 6 subjects in the study. Of the recovered [14C]-radioactivity, approximately 40% and 60% were found in the urine and feces, respectively.
Three metabolites of triamcinolone acetonide have been identified. They are 6β-hydroxytriamcinolone acetonide, 21-carboxytriamcinolone acetonide and 21-carboxy-6β-hydroxytriamcinolone acetonide. All three metabolites are expected to be substantially less active than the parent compound due to (a) the dependence of anti-inflammatory activity on the presence of a 21-hydroxyl group, (b) the decreased activity observed upon 6-hydroxylation, and (c) the markedly increased water solubility favoring rapid elimination. There appeared to be some quantitative differences in the metabolites among species. No differences were detected in metabolic pattern as a function of route of administration.
CLINICAL TRIALS
Double-blind, placebo-controlled efficacy and safety studies have been conducted in asthma patients with a range of asthma severities, from those patients with mild disease to those with severe disease requiring oral steroid therapy.
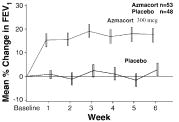
The efficacy and safety of Azmacort Inhalation Aerosol given twice daily was demonstrated in two placebo-controlled clinical trials. In two separate studies, 222 asthmatic patients were randomized to receive either Azmacort Inhalation Aerosol 300 mcg twice daily or matching placebo for a treatment period of 6 weeks. Patients were adult asthmatics who were using inhaled beta2-agonists on more than an occasional basis (at least three times weekly), either without or with inhaled corticosteroids, for control of their asthma symptoms. For the combined studies, 48% (52/109) patients randomized to placebo and 41% (46/113) patients randomized to Azmacort Inhalation Aerosol treatment were previously treated with inhaled corticosteroids.
Results of weekly lung function tests (FEV1) from one of these trials is presented graphically below. Results of the second study are presented in tabular form as the changes in asthma measures from baseline to the end of the treatment period.
| Asthma Measure | Placebo (N=61) | Azmacort 300 mcg bid (N=60) |
|
aEndpoint results are obtained from the last evaluable data, regardless of whether the patient completed 6 weeks of treatment |
||
|
bScale (0-6) with 0 = no symptom: Maximum Score (AM + PM) =12 |
||
| Percent Change in FEV1(%) | 2.8% | 17.5% |
| Increase in Morning Peak Flow Rate (L/min) | 6.7 | 45.9 |
| Decrease in Albuterol Use (puffs/day) | 0.6 | 3.4 |
| Decrease in Daily Asthma Symptom Score (units/day)b | 0.5 | 2.3 |
In both studies, treatment with Azmacort Inhalation Aerosol (300 mcg twice daily) resulted in significant improvements in all clinical asthma measures (lung functions, asthma symptoms, use of as-needed beta2-agonist medications) when compared to placebo.
INDICATIONS
Azmacort Inhalation Aerosol is indicated in the maintenance treatment of asthma as prophylactic therapy. Azmacort Inhalation Aerosol is also indicated for asthma patients who require systemic corticosteroid administration, where adding Azmacort may reduce or eliminate the need for the systemic corticosteroids.
Azmacort Inhalation Aerosol is NOT indicated for the relief of acute bronchospasm.
CONTRAINDICATIONS
Azmacort Inhalation Aerosol is contraindicated in the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required.
Hypersensitivity to triamcinolone acetonide or any of the other ingredients in this preparation contraindicates its use.
WARNINGS
Particular care is needed in patients who are transferred from systemically active corticosteroids to Azmacort Inhalation Aerosol because deaths due to adrenal insufficiency have occurred in asthmatic patients during and after transfer from systemic corticosteroids to aerosolized steroids in recommended doses. After withdrawal from systemic corticosteroids, a number of months is usually required for recovery of hypothalamic-pituitary-adrenal (HPA) function. For some patients who have received large doses of oral steroids for long periods of time before therapy with Azmacort Inhalation Aerosol is initiated, recovery may be delayed for one year or longer. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infections, particularly gastroenteritis or other conditions with acute electrolyte loss. Although Azmacort Inhalation Aerosol may provide control of asthmatic symptoms during these episodes, in recommended doses it supplies only normal physiological amounts of corticosteroidsystemically and does NOT provide the increased systemic steroid which is necessary for coping with these emergencies.
During periods of stress or a severe asthmatic attack, patients who have been recently withdrawn from systemic corticosteroids should be instructed to resume systemic steroids (in large doses) immediately and to contact their physician for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic steroids during periods of stress or a severe asthma attack.
Localized infections with Candida albicans have occurred infrequently in the mouth and pharynx. These areas should be examined by the treating physician at each patient visit. The percentage of positive mouth and throat cultures for Candida albicans did not change during a year of continuous therapy. The incidence of clinically apparent infection is low (2.5%). These infections may disappear spontaneously or may require treatment with appropriate antifungal therapy or discontinuance of treatment with Azmacort Inhalation Aerosol.
Children who are on immunosuppressant drugs are more susceptible to infections than healthy children. Chickenpox and measles, for example, can have a more serious or even fatal course in children on immunosuppressant doses of corticosteroids. In such children, or in adults who have not had these diseases, particular care should be taken to avoid exposure. If exposed, therapy with varicella zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG), as appropriate, may be indicated. If chickenpox develops, treatment with antiviral agents may be considered.
Azmacort Inhalation Aerosol is not to be regarded as a bronchodilator and is not indicated for rapid relief of bronchospasm.
As with other inhaled asthma medications, bronchospasm may occur with an immediate increase in wheezing following dosing. If bronchospasm occurs following use of Azmacort Inhalation Aerosol, it should be treated immediately with a fast-acting inhaled bronchodilator. Treatment with Azmacort Inhalation Aerosol should be discontinued and alternative treatment should be instituted.
Patients should be instructed to contact their physician immediately when episodes of asthma which are not responsive to bronchodilators occur during the course of treatment with Azmacort Inhalation Aerosol. During such episodes, patients may require therapy with systemic corticosteroids.
The use of Azmacort Inhalation Aerosol with systemic prednisone, dosed either daily or on alternate days, could increase the likelihood of HPA suppression compared to a therapeutic dose of either one alone. Therefore, Azmacort Inhalation Aerosol should be used with caution in patients already receiving prednisone treatment for any disease.
Transfer of patients from systemic steroid therapy to Azmacort Inhalation Aerosol may unmask allergic conditions previously suppressed by the systemic steroid therapy, e.g., rhinitis, conjunctivitis, and eczema.
PRECAUTIONS
Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to pediatric patients (see PRECAUTIONS, Pediatric Use). Because of the possibility of systemic absorption of inhaled corticosteroids, patients treated with these drugs should be observed carefully for any evidence of systemic corticosteroid effects including suppression of growth in children. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of a decrease in adrenal function.
During withdrawal from oral steroids, some patients may experience symptoms of systemically active steroid withdrawal, e.g., joint and/or muscular pain, lassitude, and depression, despite maintenance or even improvement of respiratory function. (See DOSAGE AND ADMINISTRATION.) Although steroid withdrawal effects are usually transient and not severe, severe and even fatal exacerbation of asthma can occur if the previous daily oral corticosteroid requirement had significantly exceeded 10 mg/day of prednisone or equivalent.
In responsive patients, inhaled corticosteroids will often permit control of asthmatic symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since triamcinolone acetonide is absorbed into the circulation and can be systemically active, the beneficial effects of Azmacort Inhalation Aerosol in minimizing or preventing HPA dysfunction may be expected only when recommended dosages are not exceeded.
Suppression of HPA function has been reported in volunteers who received 4000 mcg daily of triamcinolone acetonide by oral inhalation. In addition, suppression of HPA function has been reported in some patients who have received recommended doses for as little as 6 to 12 weeks. Since the response of HPA function to inhaled corticosteroids is highly individualized, the physician should consider this information when treating patients.
When used at excessive doses or at recommended doses in a small number of susceptible individuals, systemic corticosteroid effects such as hypercorticoidism and adrenal suppression may appear. If such changes occur, Azmacort ® Inhalation Aerosol should be discontinued slowly, consistent with accepted procedures for reducing systemic steroid therapy and for management of asthma symptoms.
Azmacort Inhalation Aerosol should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, parasitic, or viral infections; or ocular herpes simplex.
The long-term local and systemic effects of Azmacort Inhalation Aerosol in human subjects are still not fully known. While there has been no clinical evidence of adverse experiences, the effects resulting from chronic use of Azmacort Inhalation Aerosol on developmental or immunologic processes in the mouth, pharynx, trachea, and lung are unknown.
Information for Patients: Patients being treated with Azmacort Inhalation Aerosol should receive the following information and instructions. This information is intended to aid them in the safe and effective use of this medication. It is not a complete disclosure of all possible adverse or intended effects.
Patients should use Azmacort Inhalation Aerosol at regular intervals as directed. Results of clinical trials indicate that significant improvement in asthma may occur by 1 week, but maximum benefit may not be achieved for 2 weeks or more. The patient should not increase the prescribed dosage but should contact the physician if symptoms do not improve or if the condition worsens.
In clinical studies and post-marketing experience with Azmacort Inhalation Aerosol, local infections of the oropharynx with Candida albicans have occurred. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral antifungal) therapy while remaining on treatment with Azmacort Inhalation Aerosol. However, at times therapy with Azmacort Inhalation Aerosol may need to be interrupted.
Patients should be instructed to track their use of Azmacort Inhalation Aerosol and to dispose of the canister after 240 actuations since reliable dose delivery cannot be assured after 240 doses.
Patients who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles and, if exposed, to obtain medical advice.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No evidence of treatment-related carcinogenicity was demonstrated after two years of once daily gavage of triamcinolone acetonide at doses of 0.05, 0.2, and 1.0 mcg/kg (approximately 0.02, 0.07, and 0.4% of the maximum recommended human daily inhalation dose on a mcg/m2 basis) in the rat and 0.1, 0.6, and 3.0 mcg/kg (approximately 0.02, 0.1, and 0.6% of the maximum recommended human daily inhalation dose on a mcg/m2 basis) in a mouse.
Mutagenesis studies with triamcinolone acetonide have not been carried out.
No evidence of impaired fertility was manifested when oral doses of up to 15.0 mcg/kg (8% of the maximum recommended human daily inhalation dose on a mcg/m2 basis) were administered to female and male rats. However, triamcinolone acetonide at oral doses of 8 mcg/kg (approximately 4% of the maximum recommended human daily inhalation dose on a mcg/m2 basis) caused dystocia and prolonged delivery and at oral doses of 5.0 mcg/kg (approximately 2.5% of the maximum recommended human daily inhalation dose on a mcg/m2 basis) and above caused increases in fetal resorptions and stillbirths and decreases in pup body weight and survival. At a lower dose of 1.0 mcg/kg (approximately 0.5% of the maximum recommended human daily inhalation dose on a mcg/m2 basis) it did not induce the above mentioned effects.
Pregnancy: Pregnancy Category C. Triamcinolone acetonide has been shown to be teratogenic at inhalational doses of 20, 40, and 80 mcg/kg in rats (approximately 0.1, 0.2, and 0.4 times the maximum recommended human daily inhalation dose on a mcg/m2 basis, respectively), in rabbits at the same doses (approximately 0.2, 0.4, and 0.8 times the maximum recommended human daily inhalation dose on a mcg/m2 basis, respectively) and in monkeys, at an inhalational dose of 500 mcg/kg (approximately 5 times the maximum recommended human daily inhalation dose on a mcg/m2 basis). Dose related teratogenic effects in rats and rabbits included cleft palate and/or internal hydrocephaly and axial skeletal defects whereas the teratogenic effects observed in the monkey were CNS and/or cranial malformations. There are no adequate and well controlled studies in pregnant women. Triamcinolone acetonide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Experience with oral glucocorticoids since their introduction in pharmacologic as opposed to physiologic doses suggests that rodents are more prone to teratogenic effects from glucocorticoids than humans. In addition, because there is a natural increase in glucocorticoid production during pregnancy, most women will require a lower exogenous steroid dose and many will not need glucocorticoid treatment during pregnancy.
Nonteratogenic Effects: Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Such infants should be carefully observed.
Nursing Mothers: It is not known whether triamcinolone acetonide is excreted in human milk. Because other corticosteroids are excreted in human milk, caution should be exercised when Azmacort Inhalation Aerosol is administered to nursing women.
Pediatric Use: Safety and effectiveness have not been established in pediatric patients below the age of 6.
Controlled clinical studies have shown that orally inhaled corticosteroids may cause a reduction in growth velocity in pediatric patients. In these studies, the mean reduction in growth velocity was approximately one centimeter (cm) per year (range 0.3 to 1.8 cm per year; 0.12 to 0.71 inches) and appears to depend upon dose and duration of exposure. [The specific growth effects of Azmacort have also been studied in a controlled clinical trial (see data below)]. This effect was observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function.
To assess if Azmacort has an effect on growth, a one-year, randomized, open-label study of pre-pubescent boys and girls ages 6-11 with moderate to severe asthma was conducted. Children with moderate asthma were randomized to a nonsteroidal treatment or to Azmacort, children with severe asthma to Azmacort plus prednisone or just prednisone alone. A sex and age matched group of healthy non-asthmatic children was also included. The average daily dose of Azmacort was 400 mcg (range 75 to 1600 mcg/day, dose adjustments were permitted). Non-asthmatic children (mean 8.2 years) grew 5.93 cm/year (n=96). In the moderate asthma groups, the Azmacort children (mean 8.2 years) grew 5.34 cm/year (n=101) and the nonsteroidal children (mean 8.5 years) grew 6.13 cm/year (n=95). In the severe groups, the Azmacort plus prednisone children (mean 8.2 years) grew 5.46 cm/year (n=33) and the prednisone only children (mean 8.0 years) grew 5.59 cm/year (n=31). Due to low enrollment in the severe patient groups, there was insufficient power to interpret the statistical analyses on these groups.
The long-term effects of this reduction in growth velocity associated with orally inhaled corticosteroids, including the impact on final adult height, are unknown. The potential for “catch up” growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied. The growth of children and adolescents receiving orally inhaled corticosteroids, including Azmacort, should be monitored routinely (e.g. via stadiometry). The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risk associated with alternative therapies. To minimize the systemic effects of orally inhaled corticosteroids, including Azmacort , each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
Geriatric Use: Clinical studies of Azmacort Inhalation Aerosol did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The table below describes the incidence of common adverse experiences based upon three placebo-controlled, multicenter US clinical trials of 507 patients (297 female and 210 male adults (age range 18-64)). These trials included asthma patients who had previously received inhaled beta2-agonists alone, as well as those who previously required inhaled corticosteroid therapy for the control of their asthma. The patients were treated with Azmacort Inhalation Aerosol (including doses ranging from 150 to 600 mcg twice daily for 6 weeks) or placebo.
| Adverse Event | Azmacort Dose | Placebo | ||||
| 150 mcg bid (n=57) | 300 mcg bid (n=170) | 600 mcg bid (n=57) |
(n=167) |
|||
| Sinusitis | 5 (9%) | 7 (4%) | 1 (2%) | 6 (4%) | ||
| Pharyngitis | 4 (7%) | 42 (25%) | 10 (18%) | 19 (11%) | ||
| Headache | 4 (7%) | 35 (21%) | 7 (12%) | 24 (14%) | ||
| Flu Syndrome | 2 (4%) | 8 (5%) | 1 (2%) | 5 (3%) | ||
| Back Pain | 2 (4%) | 3 (2%) | 2 (4%) | 3 (2%) | ||
Adverse events that occurred at an incidence of 1-3% in the overall Azmacort Inhalation Aerosol treatment group and greater than placebo included:
Body as a whole: facial edema, pain, abdominal pain, photosensitivity
Digestive system: diarrhea, oral monilia, toothache, vomiting
Metabolic and Nutrition: weight gain
Musculoskeletal system: bursitis, myalgia, tenosynovitis
Nervous system: dry mouth
Organs of special sense: rash
Respiratory system: chest congestion, voice alteration
Urogenital system: cystitis, urinary tract infection, vaginal monilia
In older controlled clinical trials of steroid dependent asthmatics, urticaria was reported rarely. Anaphylaxis was not reported in these controlled trials. Typical steroid withdrawal effects including muscle aches, joint aches, and fatigue were noted in clinical trials when patients were transferred from oral steroid therapy to Azmacort Inhalation Aerosol. Easy bruisability was also noted in these trials.
Hoarseness, dry throat, irritated throat, dry mouth, facial edema, increased wheezing, and cough have been reported. These adverse effects have generally been mild and transient. Cases of oral candidiasis occurring with clinical use have been reported. (See WARNINGS. ) Cases of growth suppression have been reported for orally inhaled corticosteroids (see PRECAUTIONS, Pediatric Use section).
Post Marketing: In addition to adverse events reported from clinical trials, the following events have been identified during post approval use of Azmacort Inhalation Aerosol where these events were reported voluntarily from a population of unknown size, and the frequency of occurrence cannot be determined precisely. These include rare reports of anaphylaxis, cataracts, glaucoma and very rare reports of bone mineral density loss and osteoporosis, especially with prolonged use, which may lead to an increased risk of fractures.
OVERDOSAGE
There are no data available on the effects of acute or chronic overdose. However, acute overdosing with Azmacort Inhalation Aerosol is unlikely in view of the total amount of active ingredient present and the route of administration. The maximum total daily dose (1200 mcg) has been well tolerated when administered as a single dose of 16 consecutive inhalations to adult asthmatics in a controlled clinical trial. Chronic overdosage may result in signs/symptoms of hypercorticoidism. (See PRECAUTIONS. ) The risk of candidiasis could also be increased.
DOSAGE AND ADMINISTRATION
Adults: The usual recommended dosage is two inhalations (150 mcg) given three to four times a day or four inhalations (300 mcg) given twice daily. The maximal daily intake should not exceed 16 inhalations (1200 mcg) in adults. Higher initial doses (12 to 16 inhalations per day) may be considered in patients with more severe asthma.
Children 6 to 12 Years of Age: The usual recommended dosage is one or two inhalations (75 to 150 mcg) given three to four times a day or two to four inhalations (150 to 300 mcg) given twice daily. The maximal daily intake should not exceed 12 inhalations (900 mcg) in children 6 to 12 years of age. Insufficient clinical data exist with respect to the safety and efficacy of the administration of Azmacort Inhalation Aerosol to children below the age of 6. The long-term effects of inhaled steroids, including Azmacort Inhalation Aerosol, on growth are still not fully known.
Rinsing the mouth after inhalation is advised.
Different considerations must be given to the following groups of patients in order to obtain the full therapeutic benefit of Azmacort Inhalation Aerosol:
Note : In all patients, it is desirable to titrate to the lowest effective dose once asthma stability has been achieved.
Patients Not Receiving Systemic Corticosteroids: Patients who require maintenance therapy of their asthma may benefit from treatment with Azmacort Inhalation Aerosol at the doses recommended above. In patients who respond to Azmacort Inhalation Aerosol, improvement in pulmonary function is usually apparent within one to two weeks after the initiation of therapy.
Patients Maintained on Systemic Corticosteroids: Clinical studies have shown that Azmacort Inhalation Aerosol may be effective in the management of asthmatics dependent or maintained on systemic corticosteroids and may permit replacement or significant reduction in the dosage of systemic corticosteroids.
The patient's asthma should be reasonably stable before treatment with Azmacort Inhalation Aerosol is started. Initially, Azmacort Inhalation Aerosol should be used concurrently with the patient's usual maintenance dose of systemic corticosteroid. After approximately one week, gradual withdrawal of the systemic corticosteroid is started by reducing the daily or alternate daily dose. Reductions may be made after an interval of one or two weeks, depending on the response of the patient. A slow rate of withdrawal is strongly recommended. Generally, these decrements should not exceed 2.5 mg of prednisone or its equivalent. During withdrawal, some patients may experience symptoms of systemic corticosteroid withdrawal, e.g., joint and/or muscular pain, lassitude, and depression, despite maintenance or even improvement in pulmonary function. Such patients should be encouraged to continue with the inhaler but should be monitored for objective signs of adrenal insufficiency. If evidence of adrenal insufficiency occurs, the systemic corticosteroid doses should be increased temporarily and thereafter withdrawal should continue more slowly. Inhaled corticosteroids should be used with caution when used chronically in patients receiving prednisone regimens, either daily or alternate day. (See WARNINGS. )
During periods of stress or a severe asthma attack, transfer patients may require supplementary treatment with systemic corticosteroids.
Directions for Use: An illustrated leaflet of patient instructions for proper use accompanies each package of Azmacort Inhalation Aerosol.
HOW SUPPLIED
Azmacort Inhalation Aerosol contains 60 mg triamcinolone acetonide in a 20 gram package which delivers at least 240 actuations. It is supplied with a white plastic actuator, a white plastic spacer-mouthpiece and patient's leaflet of instructions: box of one. NDC 0074–3014–60. Each actuation delivers 200 mcg triamcinolone acetonide from the valve and 75 mcg from the spacer-mouthpiece under defined in vitro test conditions.
Avoid spraying in eyes.
For best results, the canister should be at room temperature before use.
Shake well before using.
CONTENTS UNDER PRESSURE. Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw canister into fire or incinerator. Keep out of reach of children unless otherwise prescribed. Store at Controlled Room Temperature 20 to 25°C (68 to 77°F) [see USP].
Note: The indented statement below is required by the Federal government's Clean Air Act for all products containing or manufactured with chlorofluorocarbons (CFCs):
WARNING: Contains CFC-12, a substance which harms public health and the environment by destroying ozone in the upper atmosphere.
A notice similar to the above WARNING has been placed in the “Information For The Patient” portion of this package insert under the Environmental Protection Agency's (EPA's) regulations. The patient's warning states that the patient should consult his or her physician if there are questions about alternatives.
©2007 Abbott Laboratories
Manufactured for:
Abbott
Laboratories
North Chicago, IL 60064 U.S.A.
INFORMATION FOR THE PATIENT
Your Guide to the
Azmacort®
(triamcinolone
acetonide)
Inhalation Aerosol
Special Delivery System
Rx Only
Your doctor has prescribed Azmacort ® (triamcinolone acetonide) Inhalation Aerosol to help control your asthma. Your Azmacort Inhalation Aerosol is one of the most efficient and easy-to-use devices available to help you take your prescribed medication. Used properly, it will effectively and reliably relieve your asthma symptoms.
To receive the maximum benefit, it is very important that you carefully read and follow all the instructions contained in this booklet for the daily use and care of your Azmacort Inhalation Aerosol.
IMPORTANT NOTE: If you've used other metered-dose inhalers before, you may expect the Azmacort Inhalation Aerosol to deliver a noticeable “blast” of medication into your mouth.
Your AZMACORT Inhalation Aerosol, however, is designed to provide a gentle mist, not a “blast,” when used.
This gentle action makes it possible for your medication to be more effectively delivered into the passageways to your lungs, with very little left to linger in your mouth. In fact, you may not even feel the medication entering your mouth, but rest assured, that is how the Azmacort Inhalation Aerosol works.
IMPORTANT: Please read all instructions in this guide carefully before using your Azmacort Inhalation Aerosol.
BEFORE STARTING TO TAKE THIS MEDICINE, TELL YOUR DOCTOR:
- If you are pregnant or intending to become pregnant.
- If you are breast-feeding a baby.
- If you are allergic to Azmacort Inhalation Aerosol or any other orally inhaled glucocorticoid.
- If you are taking other medications. In some circumstances, this medication may not be suitable and your doctor may wish to give you a different medicine.

PREPARE YOUR AZMACORT INHALATION AEROSOL
INHALER FOR USE
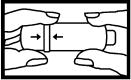
STEP 1
1. Line up the arrows on the inhaler.
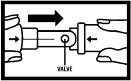
STEP 2
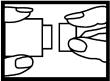
2. Gently pull the inhaler to its fully extended position. You will see the valve (small hole) where the medication will come out.
STEP 3
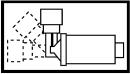
3. Adjust the inhaler into an “L” shape. It is hinged to swing in one direction only.
STEP 4
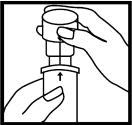
4. The ridge on the top part of the inhaler should fit into the notch on the bottom part.
STEP 5
5. Remove the mouthpiece cap. To prepare your Azmacort Inhalation Aerosol for use, the inhaler must be primed prior to the first use. To prime, hold the inhaler upright, with the mouthpiece facing away from you. Shake the inhaler gently, then press the canister firmly and quickly. Repeat this procedure again so a total of 2 puffs are released. Your Azmacort Inhalation Aerosol is now ready for use.
Repriming is only necessary when your inhaler has not been used for more than 3 days. To reprime, shake the inhaler and release one puff. Repeat this procedure again so a total of two puffs are released.
USING YOUR AZMACORT INHALATION AEROSOL INHALER
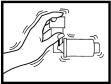
STEP 6
6. The metal Azmacort canister has already been inserted into the inhaler. Once you have opened the inhaler, shake it well before each use. IMPORTANT: You must shake the inhaler each and every time before inhaling the medication. If your doctor has instructed you to take more than one breath of medication at a time, you must shake the inhaler EACH TIME before each inhalation of medication, NOT JUST ONCE.
STEP 7
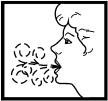
7. Breathe out to empty your lungs completely before using the inhaler! This is important to make sure that you can breathe the medication deeply into your lungs.
STEP 8
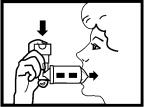
8. Place mouthpiece into your mouth, and close your lips tightly around it. Press down firmly and steadily on the metal canister while breathing in slowly and deeply THROUGH YOUR MOUTH ONLY. (If necessary, pinch your nose closed.) Be sure to release your finger pressure from the top of the canister after the medication is released.
Remember, the Azmacort Inhalation Aerosol delivers a gentle mist of medication, so don't be surprised if you hardly feel it.
Do not remove the inhaler from your mouth after breathing in the medication. Hold your breath for 10 seconds with the inhaler STILL in your mouth, THEN remove the inhaler and breathe out very slowly.
Unlike the other inhalers you may have used, you will not feel the medication impact the back of your mouth. This is because of the unique design of the Azmacort Inhalation Aerosol delivery system.

STEP 9
9. If your doctor has told you to take more than one breath of medication at a time: WAIT AT LEAST 60 SECONDS between each one, then start again at Step 6.
STEP 10
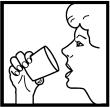
10. After the prescribed number of inhalations, thoroughly rinse out your mouth with water. NOTE: If your mouth becomes sore or develops a rash, be sure to mention this to your physician, but do not stop using your inhaler unless instructed to do so.
DOSAGE: USE ONLY AS DIRECTED BY YOUR PHYSICIAN
WARNING: Azmacort® (triamcinolone acetonide) Inhalation Aerosol contains medication that is intended for treatment of your asthma. It does not contain medication intended to provide rapid relief of your breathing difficulties during an asthma attack.
It is very important that you use Azmacort Inhalation Aerosol regularly at the intervals recommended by your doctor, and not as an emergency measure. Your physician will decide whether other medication is needed, should you require immediate relief.
CAUTION: CONTENTS OF CANISTER UNDER PRESSURE.
Do not puncture. Do not store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw canisters into a fire or incinerator. Please keep out of the reach of children.
Note: The indented statement below is required by the Federal government's Clean Air Act for all products containing or manufactured with chlorofluorocarbons (CFCs):
This product contains CFC-12, a substance which harms the environment by destroying ozone in the upper atmosphere.
Your physician has determined that this product is likely to help your personal health. USE THIS PRODUCT AS DIRECTED, UNLESS INSTRUCTED TO DO OTHERWISE BY YOUR PHYSICIAN. If you have any questions about alternatives, consult with your physician.
IMPORTANT TIPS FOR USING YOUR AZMACORT INHALATION AEROSOL INHALER
- Always use only as directed by your physician. Do not use it more often than instructed; do not skip doses.
- Follow all instructions in this booklet very closely and carefully for best results, especially those for use and cleaning.
- Remember, repriming is only necessary when the inhaler has not been used for more than 3 days. To reprime, shake the inhaler gently and release one puff. Repeat this procedure again so that a total of two puffs have been released. Do not reprime between more frequent usage.
-
Please Note:
You will receive a new Azmacort Inhalation Aerosol unit each time you refill your prescription. This is done to assure optimal working order of the unique Azmacort Inhalation Aerosol spacer device/delivery system. In addition, a new Azmacort Inhalation Aerosol will guard against a build-up of the drug on the barrel portion of the device, maximizing the cleanliness of your unit. The cost of Azmacort Inhalation Aerosol is MINIMALLY affected by including a new inhaler with each prescription.
STORING YOUR AZMACORT INHALATION AEROSOL INHALER
- Keep your inhaler out of the reach of children, unless otherwise prescribed.
- Store your Azmacort Inhalation Aerosol, including the metal canister, at room temperature.
- Protect from freezing temperatures and direct sunlight.
- For best results, the canister should be at room temperature before use.
- DO NOT use after the date shown as “EXP”on the label or box.
- Azmacort Inhalation Aerosol canisters are for use with Azmacort Inhalation Aerosol actuators and spacer-mouthpieces only. The actuator and spacer-mouthpiece should not be used with other aerosol medications.
- REMEMBER: This medicine has been prescribed for you by your doctor. DO NOT give this medicine to anyone else.
DAILY CARE OF YOUR AZMACORT INHALATION AEROSOL INHALER
Your Azmacort Inhalation Aerosol MUST be cleaned in lukewarm water only once each day to avoid build-up of medication particles in the inhaler that can block the puff of medication and interfere with proper operation. The use of soap, detergents, or disinfectants is unnecessary.
- IMPORTANT: Remove metal canister from inhaler. Pull canister straight out from inhaler and place aside. Canister must be removed for proper cleaning of inhaler.
- Pull apart remaining two plastic parts of inhaler, remove mouthpiece
cap, and gently wash in lukewarm water.
Dry thoroughly. - Snap the two plastic parts of the inhaler back together; push closed. Replace mouthpiece cap. Reinsert metal canister by gently turning while inserting. The canister should fit snugly without falling out.
HOW TO CHECK CONTENTS OF YOUR CANISTER
Shaking the canister will NOT give you a good estimate of how much Azmacort® (triamcinolone acetonide) Inhalation Aerosol is left.
We have included a convenient check-off chart to assist you in keeping track of medication puffs used. This will help assure that you receive the 240 “Full Puffs” of medication present.
FURTHER INFORMATION
- This leaflet does not contain the complete information about your medication.
- If you have any further questions, or are not sure about something, you should ask your doctor or pharmacist.
- You may want to read this leaflet again. Please DO NOT THROW IT AWAY until you have finished this canister.
©2007
Abbott Laboratories
Manufactured for:
Abbott Laboratories
North Chicago, IL 60064 U.S.A.
| Azmacort (Triamcinolone acetonide) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 06/2008Abbott Laboratories