LOPERAMIDE HYDROCHLORIDE
-
loperamide hydrochloride tablet
Premier Value
----------
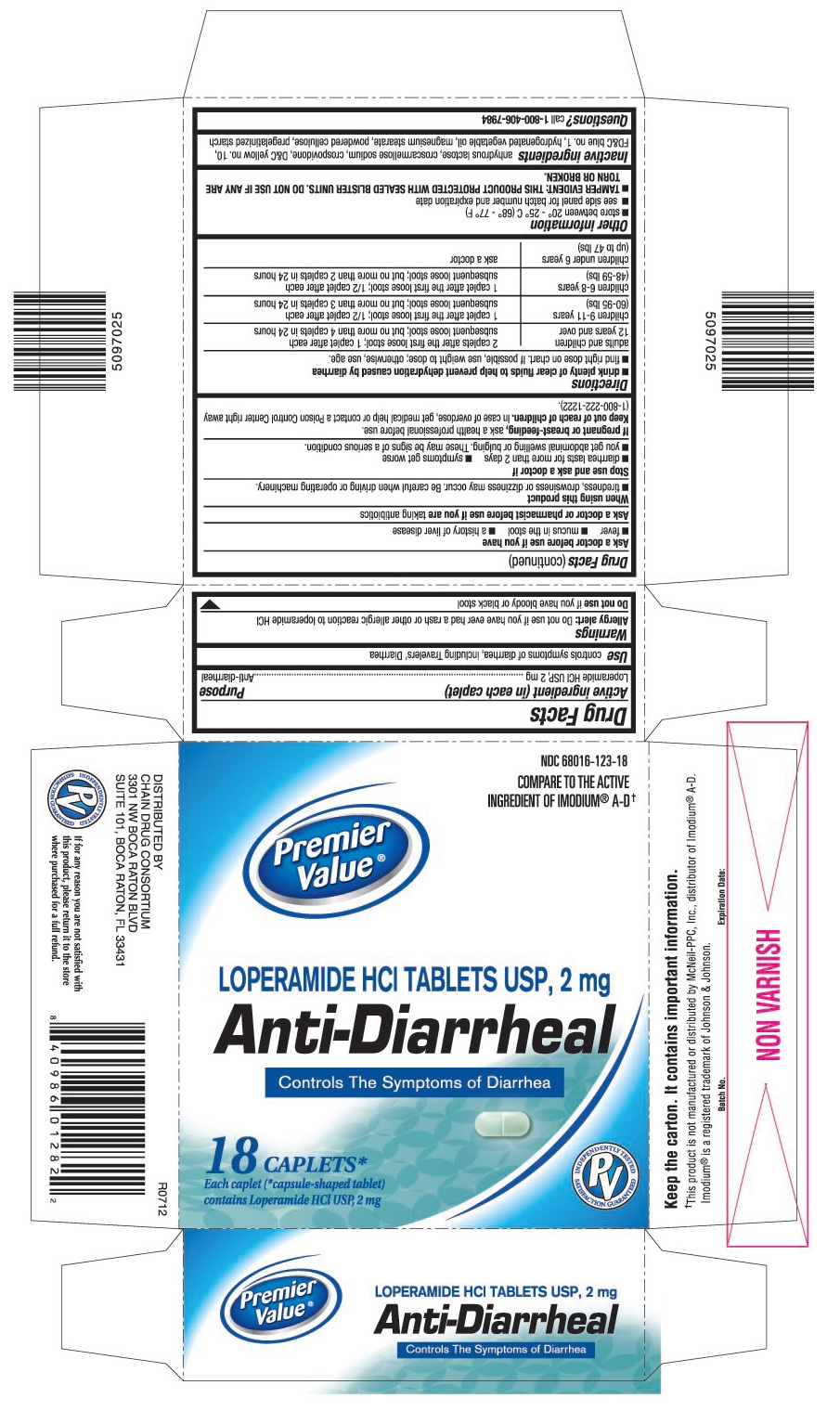
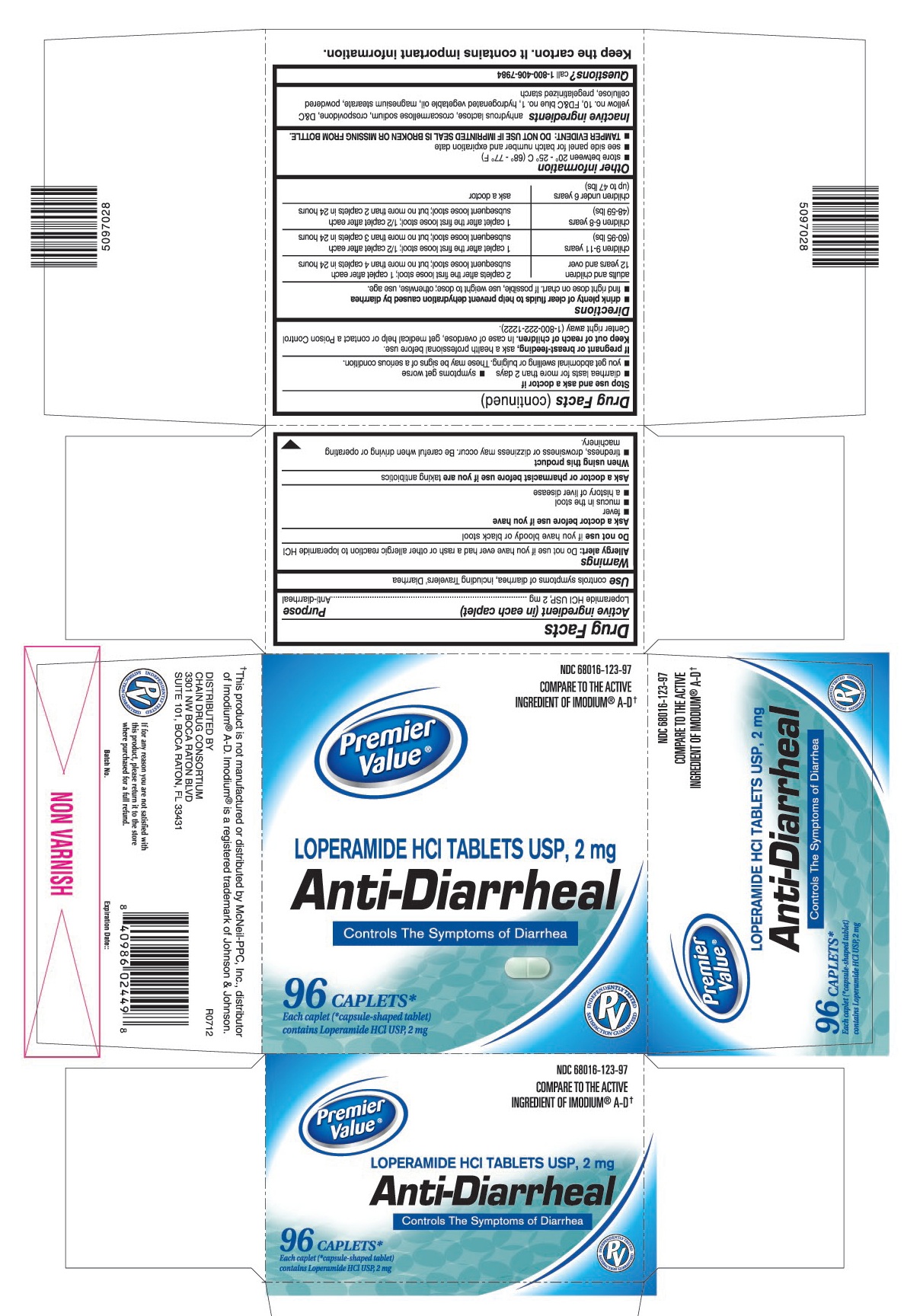
WARNINGS
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCI
When using this product
- tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
DIRECTIONS
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over | 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
| children 9-11 years (60-95 lbs) | 1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
| children 6-8 years (48-59 lbs) | 1 caplet after the first loose stool; ½ caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
| children under 6 years (up to 47 lbs) | ask a doctor |
OTHER INFORMATION
- store between 20° – 25° C (68° – 77° F)
- see side panel for lot number and expiration date
- TAMPER EVIDENT: THIS PRODUCT PROTECTED WITH SEALED BLISTER UNITS. DO NOT USE IF ANY ARE TORN OR BROKEN.
INACTIVE INGREDIENTS
anhydrous lactose, croscarmellose sodium, crospovidone, D&C yellow no.10, FD&C blue no.1, hydrogenated vegetable oil, magnesium stearate, powdered cellulose, pregelatinized starch
PRINCIPAL DISPLAY PANEL
Loperamide HCl tablets USP, 2 mg
Controls The Symptoms of Diarrhea
Each caplet (*capsule-shaped tablet) contains Loperamide HCl USP, 2 mg
COMPARE TO THE ACTIVE INGREDIENT OF IMODIUM® A-D†
This product is not manufactured or distributed by McNeil-PPC, Inc., distributor of Imodium® A-D.
| LOPERAMIDE HYDROCHLORIDE
loperamide hydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA074091 | 02/01/1993 | |
| Labeler - Premier Value (101668460) |
| Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ohm Laboratories Inc. | 051565745 | manufacture | |
Revised: 07/2012 Premier Value