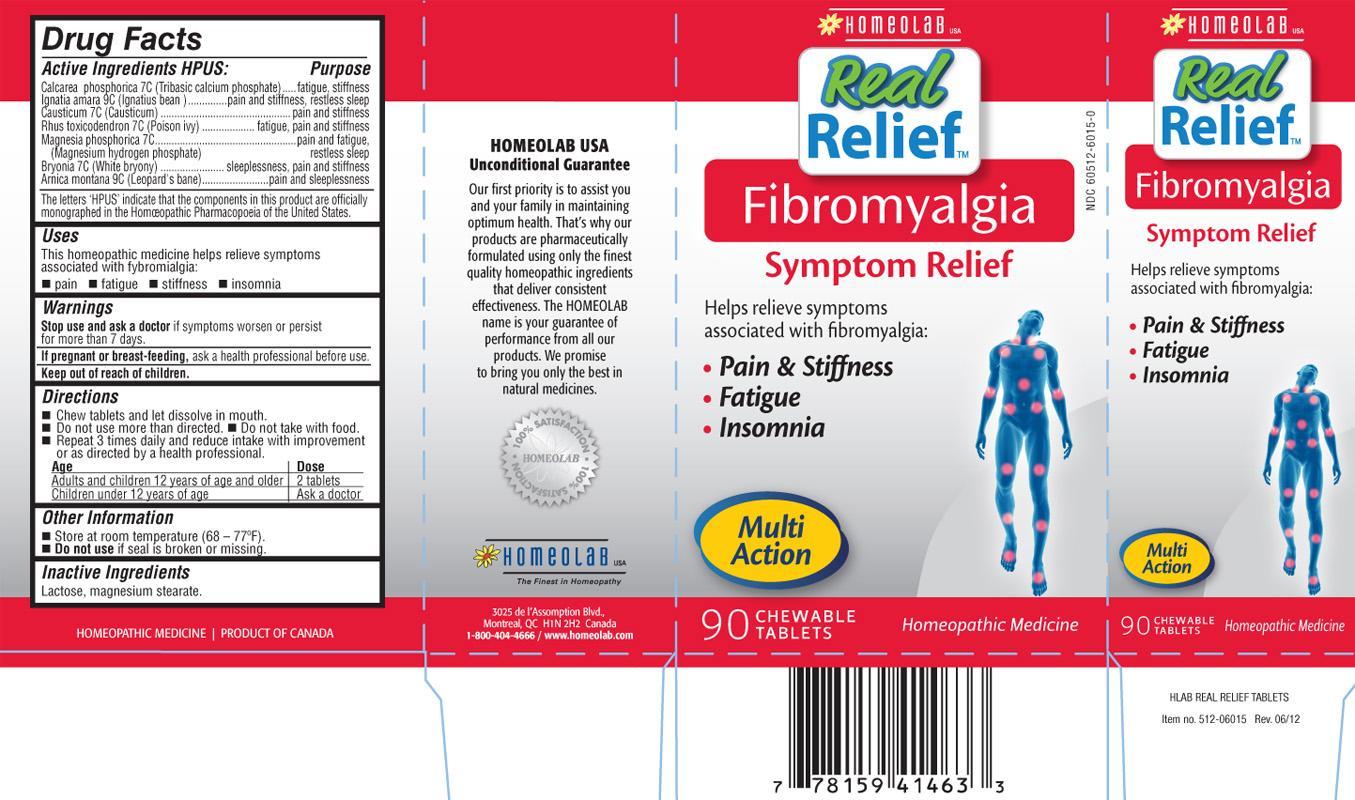
FIBROMYALGIA REAL RELIEF
-
tribasic calcium phosphate,
strychnos ignatii seed,
causticum,
toxicodendron pubescens leaf,
magnesium phosphate, dibasic trihydrate,
bryonia alba root and
arnica montana tablet, chewable
HOMEOLAB USA INC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS HPUS
Calcarea phosphorica (Tribasic calcium phosphate) 7CIgnatia amara (Ignatius bean) 9C
Causticum (Causticum) 7C
Rhus toxicodendron (Poison ivy) 7C
Magnesia phosphorica (Magnesium hydrogen phosphate) 7C
Bryonia (White bryony) 7C
Arnica montana (Leopard's bane) 9C
The letters 'HPUS' indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
PURPOSE
fatigue, stiffnesspain and stiffness, restless sleep
pain and stiffness
fatigue, pain and sleeplessness
pain and fatigue, restless sleep
sleeplessness, pain and stiffness
pain and sleeplessness
USES
This homeopathic medicine helps relieve symptoms associated with fibromialgia:
- pain
- fatigue
- stiffness
- insomnia
DIRECTIONS
Chew tablets and let dissolve in mouth.
Do not use more than directed.
Do not take with food.
Repeat 3 times daily and reduce intake with improvement or as directed by a health professional.
| Age | Dose |
|---|---|
| Adults and children 12 years of age and older | 2 tablets |
| Children under 12 years of age | Ask a doctor |
| FIBROMYALGIA
REAL RELIEF
calcarea phosphorica, ignatia amara, causticum, rhus toxicodendron, magnesia phosphorica, bryonia, arnica montana tablet, chewable |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved homeopathic | 07/25/2012 | ||
| Labeler - HOMEOLAB USA INC (202032533) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| HOMEOLAB USA INC | 202032533 | manufacture | |
Revised: 07/2012 HOMEOLAB USA INC