cetrotide (cetrorelix acetate)
[EMD Serono, Inc.]
DESCRIPTION SECTION
Cetrotide® (cetrorelix acetate for injection) is a synthetic decapeptide with gonadotropin-releasing hormone (GnRH) antagonistic activity. Cetrorelix acetate is an analog of native GnRH with substitutions of amino acids at positions 1, 2, 3, 6, and 10. The molecular formula is Acetyl-D-3-(2´-naphtyl)-alanine-D-4-chlorophenylalanine-D-3-(3´-pyridyl)-alanine-L-serine-L-tyrosine-D-citruline-L-leucine-L-arginine-L-proline-D-alanine-amide, and the molecular weight is 1431.06, calculated as the anhydrous free base. The structural formula is as follows:
Cetrorelix acetate
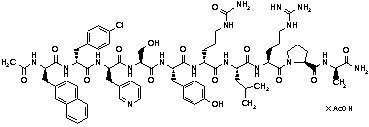
(Ac-D-Nal1-D-Cpa2-D-Pal3-Ser4-Tyr5-D-Cit6-Leu7-Arg8-Pro9-D-Ala10-NH2)
Cetrotide® (cetrorelix acetate for injection) 0.25 mg or 3 mg is a sterile lyophilized powder intended for subcutaneous injection after reconstitution with Sterile Water for Injection, USP (pH 5-8), that comes supplied in either a 1.0 mL (for 0.25 mg vial) or 3.0 mL (for 3 mg vial) pre-filled syringe. Each vial of Cetrotide® 0.25 mg (multiple dose regimen) contains 0.26-0.27 mg cetrorelix acetate, equivalent to 0.25 mg cetrorelix, and 54.80 mg mannitol. Each vial of Cetrotide® 3 mg (single dose regimen) contains 3.12-3.24 mg cetrorelix acetate, equivalent to 3 mg cetrorelix, and 164.40 mg mannitol.
CLINICAL PHARMACOLOGY SECTION
GnRH induces the production and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the gonadotrophic cells of the anterior pituitary. Due to a positive estradiol (E2) feedback at midcycle, GnRH liberation is enhanced resulting in an LH-surge. This LH-surge induces the ovulation of the dominant follicle, resumption of oocyte meiosis and subsequently luteinization as indicated by rising progesterone levels.
Cetrotide® competes with natural GnRH for binding to membrane receptors on pituitary cells and thus controls the release of LH and FSH in a dose-dependent manner. The onset of LH suppression is approximately one hour with the 3 mg dose and two hours with the 0.25 mg dose. This suppression is maintained by continuous treatment and there is a more pronounced effect on LH than on FSH. An initial release of endogenous gonadotropins has not been detected with Cetrotide®, which is consistent with an antagonist effect.
The effects of Cetrotide® on LH and FSH are reversible after discontinuation of treatment. In women, Cetrotide® delays the LH-surge, and consequently ovulation, in a dose-dependent fashion. FSH levels are not affected at the doses used during controlled ovarian stimulation. Following a single 3 mg dose of Cetrotide®, duration of action of at least 4 days has been established. A dose of Cetrotide® 0.25 mg every 24 hours has been shown to maintain the effect.
Pharmacokinetics
The pharmacokinetic parameters of single and multiple doses of Cetrotide® (cetrorelix acetate for injection) in adult healthy female subjects are summarized in Table 1.
| Table 1: Pharmacokinetic parameters of Cetrotide® following 3 mg single or 0.25 mg single and multiple (daily for 14 days) subcutaneous (sc) administration. | |||
| Single dose 3 mg | Single dose 0.25 mg | Multiple dose 0.25 mg |
|
|---|---|---|---|
|
Geometric mean (95%CIln), †arithmetic mean, or * median (min-max) |
|||
|
tmax Time to reach observed maximum plasma concentration |
|||
| t1/2 Elimination half-life | |||
| Cmax Maximum plasma concentration; multiple dose Css, max | |||
| AUC Area under the curve; single dose AUC0-inf, multiple dose AUCτ | |||
| CL Total plasma clearance | |||
| Vz Volume of distribution | |||
| a Based on iv administration (n=6, separate study 0013) | |||
| No. of subjects | 12 | 12 | 12 |
| tmax* [h] | 1.5 (0.5 – 2) | 1.0 (0.5 – 1.5) | 1.0 (0.5 – 2) |
| t1/2* [h] | 62.8 (38.2 – 108) | 5.0 (2.4 – 48.8) | 20.6 (4.1 – 179.3) |
| Cmax [ng/ml] | 28.5 (22.5 – 36.2) | 4.97 (4.17 – 5.92) | 6.42 (5.18 – 7.96) |
| AUC [ng·h/ml] | 536 (451 – 636) | 31.4 (23.4 – 42.0) | 44.5 (36.7 – 54.2) |
| CL† [ml/min·kg] | 1.28a | ||
| Vz† [l/kg] | 1.16a | ||
Absorption
Cetrotide® is rapidly absorbed following subcutaneous injection, maximal plasma concentrations being achieved approximately one to two hours after administration. The mean absolute bioavailability of Cetrotide® following subcutaneous administration to healthy female subjects is 85%.
Distribution
The volume of distribution of Cetrotide® following a single intravenous dose of 3 mg is about 1 l/kg. In vitro protein binding to human plasma is 86%.
Cetrotide® concentrations in follicular fluid and plasma were similar on the day of oocyte pick-up in patients undergoing controlled ovarian stimulation. Following subcutaneous administration of Cetrotide® 0.25 mg and 3 mg, plasma concentrations of cetrorelix were below or in the range of the lower limit of quantitation on the day of oocyte pick-up and embryo transfer.
Metabolism
After subcutaneous administration of 10 mg Cetrotide® to females and males, Cetrotide® and small amounts of (1-9), (1-7), (1-6), and (1-4) peptides were found in bile samples over 24 hours.
In in vitro studies, Cetrotide® was stable against phase I- and phase II-metabolism. Cetrotide® was transformed by peptidases, and the (1-4) peptide was the predominant metabolite.
Excretion
Following subcutaneous administration of 10 mg cetrorelix to males and females, only unchanged cetrorelix was detected in urine. In 24 hours, cetrorelix and small amounts of the (1-9), (1-7), (1-6), and (1-4) peptides were found in bile samples. 2-4% of the dose was eliminated in the urine as unchanged cetrorelix, while 5-10% was eliminated as cetrorelix and the four metabolites in bile. Therefore, only 7-14% of the total dose was recovered as unchanged cetrorelix and metabolites in urine and bile up to 24 hours. The remaining portion of the dose may not have been recovered since bile and urine were not collected for a longer period of time.
Special Populations
Pharmacokinetic investigations have not been performed either in subjects with impaired renal or liver function, or in the elderly, or in children (see PRECAUTIONS).
Pharmacokinetic differences in different races have not been determined.
There is no evidence of differences in pharmacokinetic parameters for Cetrotide® between healthy subjects and patients undergoing controlled ovarian stimulation.
Drug-Drug Interactions
No formal drug-drug interaction studies have been performed with Cetrotide® (see PRECAUTIONS).
CLINICAL STUDIES SECTION
Seven hundred thirty two (732) patients were treated with Cetrotide® (cetrorelix acetate for injection) in five (two Phase 2 dose-finding and three Phase 3) clinical trials. The clinical trial population consisted of Caucasians (95.5%) and Black, Asian, Arabian and Others (4.5%). Women were between 19 and 40 years of age (mean: 32). The studies excluded subjects with polycystic ovary syndrome (PCOS), subjects with low or no ovarian reserve, and subjects with stage III-IV endometriosis.
Two dose regimens were investigated in these clinical trials, either a single dose per treatment cycle or multiple dosing. In the Phase 2 studies, a single dose of 3 mg was established as the minimal effective dose for the inhibition of premature LH surges with a protection period of at least 4 days. When Cetrotide® is administered in a multidose regimen, 0.25 mg was established as the minimal effective dose. The extent and duration of LH-suppression is dose dependent.
In the Phase 3 program, efficacy of the single 3 mg dose regimen of Cetrotide® and the multiple 0.25 mg dose regimen of Cetrotide® was established separately in two adequate and well controlled clinical studies utilizing active comparators. A third non-comparative clinical study evaluated only the multiple 0.25 mg dose regimen of Cetrotide®. The ovarian stimulation treatment with recombinant FSH or human menopausal gonadotropin (hMG) was initiated on day 2 or 3 of a normal menstrual cycle. The dose of gonadotropins was administered according to the individual patient’s disposition and response.
In the single dose regimen study, Cetrotide® 3 mg was administered on the day of controlled ovarian stimulation when adequate estradiol levels (400 pg/ml) were obtained, usually on day 7 (range day 5-12). If hCG was not given within 4 days of the 3 mg dose of Cetrotide®, then 0.25 mg of Cetrotide® was administered daily beginning 96 hours after the 3 mg injection until and including the day of hCG administration.
In the two multiple dose regimen studies, Cetrotide® 0.25 mg was started on day 5 or 6 of COS. Both gonadotropins and Cetrotide® were continued daily (multiple dose regimen) until the injection of human chorionic gonadotropin (hCG).
Oocyte pick-up (OPU) followed by in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) as well as embryo transfer (ET) were subsequently performed. The results for Cetrotide® are summarized below in Table 2.
| Parameter | Cetrotide® 3 mg (sd, active comparator study) | Cetrotide® 0.25 mg (md, active comparator study) | Cetrotide® 0.25 mg (md, non-comparative study) |
|
|---|---|---|---|---|
| a Progesterone | ||||
| b Following initiation of Cetrotide® therapy | ||||
| c Morning values | ||||
| d Median with 5th – 95th percentiles | ||||
| e Mean ± standard deviation | ||||
| No. of subjects | 115 | 159 | 303 | |
| hCG administered [%] | 98.3 | 96.2 | 96.0 | |
| Oocyte pick-up [%] | 98.3 | 94.3 | 93.1 | |
| LH-surge [%] (LH ≥10 U/L and Pa ≥ 1 ng/mL)b | 0.0 | 1.9 | 1.0 | |
| Serum E2 [pg/ml] at day hCGc,d | 1125 (470 – 2952) | 1064 (341 – 2531) | 1185 (311 – 3676) |
|
| Serum LH [U/L] at day hCGc,d | 1.0 (0.5 – 2.5) | 1.5 (0.5 – 7.6) | 1.1 (0.5 – 3.5) |
|
| No. of follicles ≥ 11 mm at day hCGe | 11.2 ± 5.5 | 10.8 ± 5.2 | 10.4 ± 4.5 | |
| No. of oocytes: | IVFe
ICSIe | 9.2 ± 5.2 10.0 ± 4.2 | 7.6 ± 4.3 10.1 ± 5.6 | 8.5 ± 5.1 9.3 ± 5.9 |
| Fertilization rate: | IVFe
ICSIe | 0.48 ± 0.33 0.66 ± 0.29 | 0.62 ± 0.26 0.63 ± 0.29 | 0.60 ± 0.26 0.61 ± 0.25 |
| No. of embryos transferrede | 2.6 ± 0.9 | 2.1 ± 0.6 | 2.7 ± 1.0 | |
| Clinical pregnancy rate [%] per attempt per subject with ET |
22.6 26.3 |
20.8 24.1 |
19.8 23.3 |
|
In addition to IVF and ICSI, one pregnancy was obtained after intrauterine insemination. In the five Phase 2 and Phase 3 clinical trials, 184 pregnancies have been reported out of a total of 732 patients (including 21 pregnancies following the replacement of frozen-thawed embryos).
In the 3 mg regimen, 9 patients received an additional dose of 0.25 mg of Cetrotide® and two other patients received two additional doses of 0.25 mg Cetrotide®. The median number of days of Cetrotide® multiple dose treatment was 5 (range 1-15) in both studies.
No drug related allergic reactions were reported from these clinical studies.
INDICATIONS & USAGE SECTION
Cetrotide® (cetrorelix acetate for injection) is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian stimulation.
CONTRAINDICATIONS SECTION
Cetrotide® (cetrorelix acetate for injection) is contraindicated under the following conditions:
-
Hypersensitivity to cetrorelix acetate, extrinsic peptide hormones or mannitol.
-
Known hypersensitivity to GnRH or any other GnRH analogs.
-
Known or suspected pregnancy, and lactation (see PRECAUTIONS).
-
Severe renal impairment.
WARNINGS SECTION
Cetrotide® (cetrorelix acetate for injection) should be prescribed by physicians who are experienced in fertility treatment. Before starting treatment with Cetrotide®, pregnancy must be excluded (see CONTRAINDICATIONS and PRECAUTIONS).
PRECAUTIONS SECTION
GENERAL PRECAUTIONS SECTION
Cases of hypersensitivity reactions, including anaphylactoid reactions with the first dose, have been reported during post-marketing surveillance (see ADVERSE REACTIONS). A severe anaphylactic reaction associated with cough, rash, and hypotension, was observed in one patient after seven months of treatment with Cetrotide® (10 mg/day) in a study for an indication unrelated to infertility.
Special care should be taken in women with signs and symptoms of active allergic conditions or known history of allergic predisposition. Treatment with Cetrotide® is not advised in women with severe allergic conditions.
INFORMATION FOR PATIENTS SECTION
Prior to therapy with Cetrotide® (cetrorelix acetate for injection), patients should be informed of the duration of treatment and monitoring procedures that will be required. The risk of possible adverse reactions should be discussed (see ADVERSE REACTIONS). Cetrotide® should not be prescribed if a patient is pregnant.
If Cetrotide® is prescribed to patients for self-administration, information for proper use is given in the Patient Leaflet (see below).
LABORATORY TESTS SECTION
After the exclusion of preexisting conditions, enzyme elevations (ALT, AST, GGT, alkaline phosphatase) were found in 1-2% of patients receiving Cetrotide® during controlled ovarian stimulation. The elevations ranged up to three times the upper limit of normal. The clinical significance of these findings was not determined.
During stimulation with human menopausal gonadotropin, Cetrotide® had no notable effects on hormone levels aside from inhibition of LH surges.
DRUG INTERACTIONS SECTION
No formal drug interaction studies have been performed with Cetrotide®.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY SECTION
Long-term carcinogenicity studies in animals have not been performed with cetrorelix acetate. Cetrorelix acetate was not genotoxic in vitro (Ames test, HPRT test, chromosome aberration test) or in vivo (chromosome aberration test, mouse micronucleus test). Cetrorelix acetate induced polyploidy in CHL-Chinese hamster lung fibroblasts, but not in V79-Chinese hamster lung fibroblasts, cultured peripheral human lymphocytes or in an in vitro micronucleus test in the CHL-cell line. Treatment with 0.46 mg/kg cetrorelix acetate for 4 weeks resulted in complete infertility in female rats which was reversed 8 weeks after cessation of treatment.
PREGNANCY SECTION
Pregnancy Category X (see CONTRAINDICATIONS)
Cetrotide® is contraindicated in pregnant women.
When administered to rats for the first seven days of pregnancy, cetrorelix acetate did not affect the development of the implanted conceptus at doses up to 38 µg/kg (approximately 1 times the recommended human therapeutic dose based on body surface area). However, a dose of 139 μg/kg (approximately 4 times the human dose) resulted in a resorption rate and a postimplantation loss of 100%.
When administered from day 6 to near term to pregnant rats and rabbits, very early resorptions and total implantation losses were seen in rats at doses from 4.6 µg/kg (0.2 times the human dose) and in rabbits at doses from 6.8 µg/kg (0.4 times the human dose). In animals that maintained their pregnancy, there was no increase in the incidence of fetal abnormalities.
The fetal resorption observed in animals is a logical consequence of the alteration in hormonal levels affected by the antigonadotrophic properties of Cetrotide®, which could result in fetal loss in humans as well. Therefore, this drug should not be used in pregnant women.
NURSING MOTHERS SECTION
It is not known whether Cetrotide® is excreted in human milk. Because many drugs are excreted in human milk, and because the effects of Cetrotide® on lactation and/or the breast-fed child have not been determined, Cetrotide® should not be used by nursing mothers.
GERIATRIC USE SECTION
Cetrotide® is not intended to be used in subjects aged 65 and over.
ADVERSE REACTIONS SECTION
The safety of Cetrotide® (cetrorelix acetate for injection) in 949 patients undergoing controlled ovarian stimulation in clinical studies was evaluated. Women were between 19 and 40 years of age (mean: 32). 94.0 % of them were Caucasian. Cetrotide® was given in doses ranging from 0.1 mg to 5 mg as either a single or multiple dose.
Table 3 shows systemic adverse events, reported in clinical studies without regard to causality, from the beginning of Cetrotide® treatment until confirmation of pregnancy by ultrasound at an incidence ≥1% in Cetrotide® treated subjects undergoing COS.
| Table 3: Adverse Events in ≥1% | Cetrotide® N=949 |
|---|---|
| (WHO preferred term) | % (n) |
| # Intensity moderate or severe, or WHO Grade II or III, respectively | |
| Ovarian Hyperstimulation Syndrome# | 3.5 (33) |
| Nausea | 1.3 (12) |
| Headache | 1.1 (10) |
Local site reactions (e.g. redness, erythema, bruising, itching, swelling and pruritus) were reported. Usually, they were of a transient nature, mild intensity and short duration. During post-marketing surveillance, rare cases of hypersensitivity reactions including anaphylactoid reactions have been reported.
Two stillbirths were reported in Phase 3 studies of Cetrotide®.
Congenital Anomalies
Clinical follow-up studies of 316 newborns of women administered Cetrotide® were reviewed. One infant of a set of twin neonates was found to have anencephaly at birth and died after four days. The other twin was normal. Developmental findings from ongoing baby follow-up included a child with a ventricular septal defect and another child with bilateral congenital glaucoma.
Four pregnancies that resulted in therapeutic abortion in Phase 2 and Phase 3 controlled ovarian stimulation studies had major anomalies (diaphragmatic hernia, trisomy 21, Klinefelter syndrome, polymalformation, and trisomy 18). In three of these four cases, intracytoplasmic sperm injection (ICSI) was the fertilization method employed; in the fourth case, in vitro fertilization (IVF) was the method employed.
The minor congenital anomalies reported include: supernumerary nipple, bilateral strabismus, imperforate hymen, congenital nevi, hemangiomata, and QT syndrome.
The causal relationship between the reported anomalies and Cetrotide® is unknown. Multiple factors, genetic and others (including, but not limited to ICSI, IVF, gonadotropins, and progesterone) make causal attribution difficult to study.
OVERDOSAGE SECTION
There have been no reports of overdosage with Cetrotide® 0.25 mg or 3 mg in humans. Single doses up to 120 mg Cetrotide® have been well tolerated in patients treated for other indications without signs of overdosage.
DOSAGE & ADMINISTRATION SECTION
Ovarian stimulation therapy with gonadotropins (FSH, hMG) is started on cycle Day 2 or 3. The dose of gonadotropins should be adjusted according to individual response. Cetrotide® (cetrorelix acetate for injection) may be administered subcutaneously either once daily (0.25 mg dose) or once (3 mg dose) during the early- to mid-follicular phase.
In the single dose regimen, 3 mg of Cetrotide® is administered when the serum estradiol level is indicative of an appropriate stimulation response, usually on stimulation day 7 (range day 5-9). If hCG has not been administered within four days after injection of Cetrotide® 3 mg, Cetrotide® 0.25 mg should be administered once daily until the day of hCG administration.
In the multiple dose regimen, 0.25 mg of Cetrotide® is administered on either stimulation day 5 (morning or evening) or day 6 (morning) and continued daily until the day of hCG administration.
When assessment by ultrasound shows a sufficient number of follicles of adequate size, hCG is administered to induce ovulation and final maturation of the oocytes. No hCG should be administered if the ovaries show an excessive response to the treatment with gonadotropins to reduce the chance of developing ovarian hyperstimulation syndrome (OHSS).
Administration
Cetrotide® 0.25 mg and 3 mg can be administered by the patient herself after appropriate instructions by her doctor.
Directions for using Cetrotide® 0.25 mg and 3 mg with the enclosed needles and pre-filled syringe:
-
Wash hands thoroughly with soap and water.
-
Flip off the plastic cover of the vial and wipe the aluminum ring and the rubber stopper with an alcohol swab.
-
Twist the injection needle with the yellow mark (20 gauge) on the pre-filled syringe.
-
Push the needle through the rubber stopper of the vial and slowly inject the solvent into the vial.
-
Leaving the syringe on the vial, gently swirl the vial until the solution is clear and without residues. Avoid forming bubbles.
-
Draw the total contents of the vial into the syringe. If necessary, invert the vial and pull back the needle as far as needed to withdraw the entire contents of the vial.
-
Replace the needle with the yellow mark by the injection needle with the grey mark (27 gauge).
-
Invert the syringe and push the plunger until all air bubbles have been expelled.
-
Choose an injection site at the lower abdominal area, preferably around, but staying at least one inch away from the navel. If you are on a multiple dose (0.25 mg) regimen, choose a different injection site each day to minimize local irritation. Use the second alcohol swab to clean the skin at the injection site and allow alcohol to dry. Gently pinch up the skin surrounding the site of injection.
-
Insert the prescribed dose as directed by your doctor, nurse of pharmacist.
-
Use the syringe and needles only once. Dispose of the syringe and needles properly after use. If available, use a medical waste container for disposal.
HOW SUPPLIED SECTION
Cetrotide® (cetrorelix acetate for injection) 0.25 mg is available in a carton of one packaged tray (NDC 44087-1225-1). Each packaged tray contains: one glass vial containing 0.26 - 0.27 mg cetrorelix acetate (corresponding to 0.25 mg cetrorelix), one pre-filled glass syringe with 1 mL of Sterile Water for Injection, USP (pH 5-8), one 20 gauge needle (yellow), one 27 gauge needle (grey), and two alcohol swabs.
Cetrotide® (cetrorelix acetate for injection) 3 mg is available in a carton of one packaged tray (NDC 44087-1203-1). Each packaged tray contains: one glass vial containing 3.12 - 3.24 mg cetrorelix acetate (corresponding to 3 mg cetrorelix), one pre-filled glass syringe with 3 mL of Sterile Water for Injection, USP (pH 5-8), one 20 gauge needle (yellow), one 27 gauge needle (grey), and two alcohol swabs.
Storage
Cetrotide® 3 mg: Store at 25 ºC (77 ºF); excursions permitted to 15-30 ºC (59-86 ºF) [see USP Controlled Room Temperature]. Store the packaged tray in the outer carton.
Cetrotide® 0.25 mg: Store refrigerated, 2-8 ºC (36-46 ºF). Store the packaged tray in the outer carton.
Rx only
Manufactured for:
EMD Serono, Inc., Rockland, MA 02370
April 2008
1030922
SUPPLEMENTAL PATIENT MATERIAL SECTION
SPL PATIENT PACKAGE INSERT SECTION
Patient Leaflet
Cetrotide® 0.25 mg and 3 mg
Active ingredient: cetrorelix acetate
Summary
Cetrotide® blocks the effects of a natural hormone, called gonadotropin-releasing hormone (GnRH). GnRH controls the secretion of another hormone, called luteinizing hormone (LH), which induces ovulation during the menstrual cycle. During hormone treatment for ovarian stimulation, premature ovulation may lead to eggs that are not suitable for fertilization. Cetrotide® blocks such undesirable premature ovulation.
Uses
Cetrotide® is used to prevent premature ovulation during controlled ovarian stimulation.
General Cautions
Do not use Cetrotide® if you
-
have kidney disease
-
are allergic to cetrorelix acetate, mannitol or exogenous peptide hormones (medicines similar to Cetrotide®) or
-
are pregnant, or think that you might be pregnant, or if you are breast-feeding.
Consult your doctor before taking Cetrotide® if you have had severe allergic reactions.
Proper Use
Ovarian stimulation therapy is started on cycle Day 2 or 3. Cetrotide® is injected under the skin either once daily (0.25 mg dose) or once (3 mg dose), as directed by your physician. When an ultrasound examination shows that you are ready, another drug (hCG) is injected to induce ovulation.
How should you use Cetrotide®?
You may self-inject Cetrotide® after special instruction from your doctor.
To fully benefit from Cetrotide®, please read carefully and follow the instructions given below, unless your doctor advises you otherwise.
Cetrotide® is for injection under the skin of the lower abdominal area, preferably around, but staying at least one inch away from the belly button. If you are on a multiple dose (0.25 mg) regimen, choose a different injection site each day to minimize local irritation.
Dissolve Cetrotide® powder only with the water contained in the pre-filled syringe. Do not use a Cetrotide® solution if it contains particles or if it is not clear.
Before you inject Cetrotide® yourself, please read the following instructions carefully:
Directions for using Cetrotide® 0.25 mg or 3 mg with the enclosed needles and pre-filled syringe:
| 1. Wash your hands well with soap and water. |  |
||
| 2. On a clean surface, lay out everything you need (one vial of powder, one pre-filled syringe, one injection needle with a yellow mark, one injection needle with a grey mark, and two alcohol wipes). |  |
||
| 3. Flip off the plastic cover of the vial. Wipe the aluminum ring and the rubber stopper with an alcohol wipe. |  | 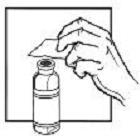 |
|
| 4. Take the injection needle with the yellow mark and remove the wrapping. Take the pre-filled syringe and remove the cover. Twist the needle on the syringe and remove the cover of the needle. | 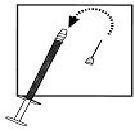 |
||
| 5. Push the needle through the center of the rubber stopper of the vial. Inject the water into the vial by slowly pushing down on the plunger of the syringe. | 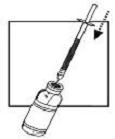 |
||
| 6. Leave the syringe on the vial. Gently shake the vial until the solution is clear and without residue. Avoid forming bubbles during dissolution. | 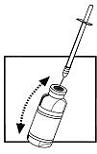 |
||
| 7. Draw the total contents of the vial into the syringe. If liquid is left in the vial, invert the vial, pull back the needle until the opening of the needle is just inside the stopper. If you look from the side through the gap in the stopper, you can control the movement of the needle and the liquid. It is important to withdraw the entire contents of the vial. | 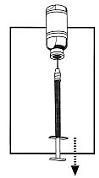 |
||
| 8. Detach the syringe from the needle and lay down the syringe. Take the injection needle with the grey mark and remove its wrapping. Twist the needle on the syringe and remove the cover of the needle. | 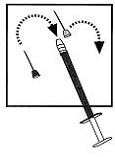 |
||
| 9. Invert the syringe and push the plunger until all air bubbles have been pushed out. Do not touch the needle or allow the needle to touch any surface. | 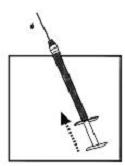 |
||
| 10. Choose an injection site at the lower abdominal area, preferably around, but at least one inch away from the belly button. If you are on a multiple dose (0.25 mg) regimen, choose a different injection site each day to minimize local irritation. Take the second alcohol wipe and clean the skin at the injection site and allow alcohol to dry. Inject the prescribed dose as directed by your doctor, nurse or pharmacist. | 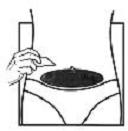 |  |
|
| 11. Use the syringe and needles only once. Dispose of the syringe and needles immediately after use (put the covers on the needles to avoid injury). A medical waste container should be used for disposal. |  |
||
SPECIAL ADVICE
What do you do if you have used too much Cetrotide®?
Contact your doctor in case of overdosage immediately to check whether an adjustment of the further ovarian stimulation procedure is required.
Possible Side Effects
Mild and short lasting reactions may occur at the injection site like reddening, itching, and swelling. Nausea and headache have also been reported.
Call your doctor if you have any side effect not mentioned in this leaflet or if you are unsure about the effect of this medicine.
Storage
How is Cetrotide® to be stored?
Store Cetrotide® in a cool dry place protected from excess moisture and heat. Store Cetrotide® 3 mg at 25 °C (77 °F). Excursions are permitted to 15-30 ºC (59-86 ºF). Store Cetrotide® 0.25 mg in the refrigerator at 2-8 °C (36-46 °F). Keep the packaged tray in the outer carton in order to protect it from light.
How long may Cetrotide® be stored?
Do not use the Cetrotide® powder or the pre-filled syringe after the expiration date, which is printed on the labels and on the carton, and dispose of the vial and the syringe properly.
How long can you keep Cetrotide® after preparation of the solution?
The solution should be used immediately after preparation.
Store the medicine out of the reach of children.
If you suspect that you may have taken more than the prescribed dose of this medicine, contact your doctor immediately. This medicine was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
This leaflet provides a summary of the information about Cetrotide®. Medicines are sometimes prescribed for uses other than those listed in the Leaflet. If you have any questions or concerns, or want more information about Cetrotide®, contact your doctor or pharmacist.
This Leaflet has been approved by the U.S. Food and Drug Administration.
June 2004
1030922
| Cetrotide (cetrorelix acetate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Cetrotide (cetrorelix acetate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 04/2008EMD Serono, Inc.