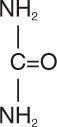KEROL AD
-
urea emulsion
PharmaDerm, A division of Fougera Pharmaceuticals Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION
Each mL of KerolTM AD contains 45% urea in a formulation consisting of: propylene glycol, caprylic/capric triglycerides, polyethylene glycol 300, purified water, trolamine, vitamin E acetate, lactic acid, glycerin, polysorbate-60, linoleic acid, sorbitan monostearate, zinc undecylenate, titanium dioxide, cetyl alcohol, EDTA disodium, hydroxyethyl cellulose and xanthan gum.
Urea is a diamide of carbonic acid with the following chemical structure:
CLINICAL PHARMACOLOGY
Urea gently dissolves the intercellular matrix, which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas.
INDICATIONS AND USES
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis pilaris, keratosis palmaris, keratoderma, corns and calluses.
PRECAUTIONS
This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use.
PREGNANCY
Pregnancy Category B. Animal reproduction studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, KerolTM AD should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether or not this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when KerolTM AD is administered to a nursing woman.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
ADVERSE REACTIONS
Transient stinging, burning, itching or irritation may occur and normally disappear on discontinuing the medication.
DOSAGE AND ADMINISTRATION
Apply KerolTM AD to affected skin twice per day, or as directed by a physician. Rub in until completely absorbed.
HOW SUPPLIED
KerolTM AD (45% urea emulsion) is supplied as:
240 mL bottle, NDC 10337-643-24
Store at 15˚-30˚ C (59˚- 86˚ F).
Protect from freezing.
Mfd. for:
PharmaDerm®
A division of Nycomed US Inc.
Melville, NY 11747 USA
www.pharmaderm.com
Mfd. by:
Pegasus Laboratories, Inc.
Pensacola, FL 32514
IF643
R5/09
How to use
Rx only
Kerol™ AD
(45% urea emulsion)
Accu-Dose
In a zinc undecylenate and lactic acid vehicle
Steps for using Kerol AD to treat dry skin conditions including psoriasis, xerosis, ichthyosis, keratosis pilaris, keratosis palmaris, keratoderma, dermatitis, pruritus, eczema, corns and calluses.
Kerol AD can be applied directly to the treatment area(s) without pouring from a bottle or squeezing from a tube. The pump is designed to consistently deliver a precise amount of Kerol AD with each use once the pump has been primed and medication is delivered to the spout. To begin using Kerol AD:
- Remove the cap.
- With the first use, prime the chamber by pushing the pump top all the way down and then release. Repeat this four to six times until the chamber inside is filled and the Kerol AD passes through the spout.*
- To use after the chamber is filled, push the pump top all the way down to dispense one dose of Kerol AD. A precise amount of Kerol AD will be consistently delivered with each use.
- Apply Kerol AD to the affected area(s).
- Gently massage Kerol AD into the affected area twice a day or as directed by your physician. Keep Kerol AD away from the eyes, lips or mucous membranes.
- Replace the cap.
* Sample bottles of Kerol AD have a different fill ratio and will need to be pumped eight to ten times to prime the chamber and get the Kerol AD to pass through the spout.
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 240 mL CONTAINER LABEL
NDC 10337-643-24
PharmaDerm®
NDC 10337-643-24
Rx only
KEROL™ AD
(45% urea emulsion)
Accu-Dose
In a zinc undecylenate and lactic acid vehicle
For Topical Use Only
Net Content 240 mL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 240 mL CARTON
PharmaDerm®
NDC 10337-643-24
Rx only
KEROL™ AD
(45% urea emulsion)
Accu-Dose
In a zinc undecylenate and
lactic acid vehicle
For Topical
Use Only
Net Content 240 mL
| KEROL AD
urea emulsion |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 09/15/2009 | 05/31/2012 | |
| Labeler - PharmaDerm, A division of Fougera Pharmaceuticals Inc. (043838424) |
Revised: 07/2012 PharmaDerm, A division of Fougera Pharmaceuticals Inc.