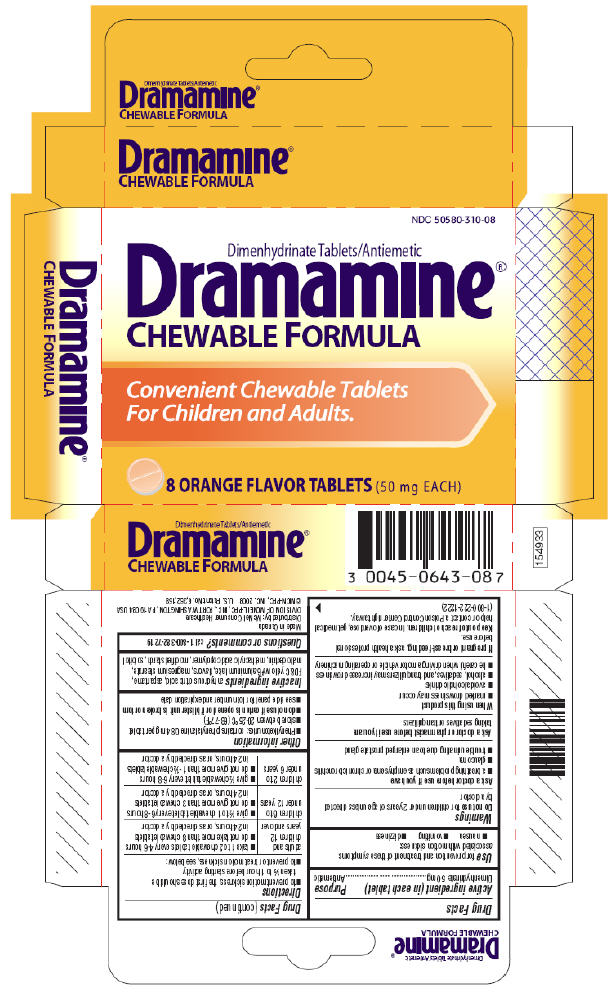
DRAMAMINE CHEWABLE FORMULA- dimenhydrinate tablet, chewable
McNeil Consumer Healthcare Div McNeil-PPC, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Use
for prevention and treatment of these symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- to prevent motion sickness, the first dose should be taken ½ to 1 hour before starting activity
- to prevent or treat motion sickness, see below:
| adults and children 12 years and over |
|
| children 6 to under 12 years |
|
| children 2 to under 6 years |
|
Other information
- Phenylketonurics: contains phenylalanine 0.84 mg per tablet
- store between 20–25°C (68–77°F)
- do not use if carton is opened or if blister unit is broken or torn
- see side panel for lot number and expiration date
| DRAMAMINE
CHEWABLE FORMULA
dimenhydrinate tablet, chewable |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - McNeil Consumer Healthcare Div McNeil-PPC, Inc (878046358) |
Revised: 04/2012
Document Id: f1dc68e5-01c4-456e-97f0-0776308a78a3
Set id: 88c564ee-3687-4c5c-8dd1-65857dec3621
Version: 3
Effective Time: 20120430
McNeil Consumer Healthcare Div McNeil-PPC, Inc