IMOVAX RABIES
-
rabies virus strain pm-1503-3m antigen (propiolactone inactivated) and water
Sanofi Pasteur Inc.
----------
DESCRIPTION
The Imovax® Rabies Vaccine produced by Sanofi Pasteur SA is a sterile, stable, freeze-dried suspension of rabies virus prepared from strain PM-1503-3M obtained from the Wistar Institute, Philadelphia, PA.
The virus is harvested from infected human diploid cells, MRC-5 strain, concentrated by ultrafiltration and is inactivated by beta-propiolactone. One dose of reconstituted vaccine contains less than 100 mg albumin, less than 150 µg neomycin sulfate and 20 µg of phenol red indicator. This vaccine must only be used intramuscularly and as a single dose vial.
The vaccine contains no preservative or stabilizer. It should be used immediately after reconstitution, and if not administered promptly, discard contents.
The potency of one dose (1.0 mL) Sanofi Imovax Rabies Vaccine is equal to or greater than 2.5 international units of rabies antigen.
CLINICAL PHARMACOLOGY
Pre-exposure immunization
High titer antibody responses of the Sanofi Pasteur SA Imovax Rabies Vaccine made in human diploid cells have been demonstrated in trials conducted in England1, Germany2,3, France4 and Belgium.5 Seroconversion was often obtained with only one dose. With two doses one month apart, 100% of the recipients developed specific antibody and the geometric mean titer of the group was approximately 10 international units. In the US, Sanofi Pasteur SA Imovax Rabies Vaccine resulted in geometric mean titers (GMT) of 12.9 IU/mL at Day 49 and 5.1 IU/mL at Day 90 when three doses were given intramuscularly during the course of one month. The range of antibody responses was 2.8 to 55.0 IU/mL at Day 49 and 1.8 to 12.4 IU at Day 90.6 The definition of a minimally accepted antibody titer varies among laboratories and is influenced by the type of test conducted. CDC currently specifies a 1:5 titer (complete inhibition) by the rapid fluorescent focus inhibition test (RFFIT) as acceptable. The World Health Organization (WHO) specifies a titer of 0.5 IU.
Postexposure immunization
Postexposure efficacy of Sanofi Pasteur SA Imovax Rabies Vaccine was successfully proven during clinical experience in Iran7 in conjunction with antirabies serum. Forty-five persons severely bitten by rabid dogs and wolves received Sanofi vaccine within hours of and up to 14 days after the bites. All individuals were fully protected against rabies.
There have been reports of possible vaccine failure when the vaccine has been administered in the gluteal area. Presumably subcutaneous fat in the gluteal area may interfere with the immunogenicity of human diploid cell rabies vaccine (HDCV).26,29 For adults and children, Rabies Vaccine should be administered in the deltoid muscle. (See DOSAGE AND ADMINISTRATION.)
INDICATIONS AND USAGE
1. Rationale of treatment
Physicians must evaluate each possible rabies exposure. Local or state public health officials should be consulted if questions arise about the need for prophylaxis.8
In the United States and Canada, the following factors should be considered before antirabies treatment is initiated.
Species of biting animal
Carnivorous wild animals (especially skunks, raccoons, foxes, coyotes, and bobcats) and bats are the animals most commonly infected with rabies and have caused most of the indigenous cases of human rabies in the United States since 1960. Unless an animal is tested and shown not to be rabid, postexposure prophylaxis should be initiated upon bite or nonbite exposure to the animals. (See definition in "Type of Exposure" below.) If treatment has been initiated and subsequent testing in a competent laboratory shows the exposing animal is not rabid, treatment can be discontinued.8
The likelihood that a domestic dog or cat is infected with rabies varies from region to region; hence the need for postexposure prophylaxis also varies.8
Rodents (such as squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, and mice) and lagomorphs (including rabbits and hares) are rarely found to be infected with rabies and have not been known to cause human rabies in the United States. In these cases, the state or local health department should be consulted before a decision is made to initiate postexposure antirabies prophylaxis.8
Circumstances of biting incident
An UNPROVOKED attack is more likely than a provoked attack to indicate the animal is rabid. Bites inflicted on a person attempting to feed or handle an apparently healthy animal should generally be regarded as PROVOKED.
Type of exposure
Rabies is transmitted by introducing the virus into open cuts or wounds in skin or via mucous membranes. The likelihood of rabies infection varies with the nature and extent of exposure. Two categories of exposure should be considered.
Nonbite
Scratches, abrasions, open wounds, or mucous membranes contaminated with saliva or other potentially infectious material, such as brain tissue, from a rabid animal. Casual contact, such as petting a rabid animal (without a bite or nonbite exposure as described above), does not constitute an exposure and is not an indication for prophylaxis. There have been two instances of airborne rabies acquired in laboratories and two probable airborne rabies cases acquired in a bat-infested cave in Texas.8,9
The only documented cases for rabies from human-to-human transmission occurred in four patients in the United States and overseas who received corneas transplanted from persons who died of rabies undiagnosed at the time of death.9,10 Stringent guidelines for acceptance of donor corneas should reduce this risk. Bite and nonbite exposure from humans with rabies theoretically could transmit rabies, although no cases of rabies acquired this way have been documented. Each potential exposure to human rabies should be carefully evaluated to minimize unnecessary rabies prophylaxis.8,11
2. Pre- and postexposure treatment of rabies
A. Pre-exposure
See Table 1
Pre-exposure immunization may be offered to persons in high-risk groups, such as veterinarians, animal handlers, certain laboratory workers, and persons spending time (eg, 1 month or more) in foreign countries where rabies is a constant threat. Persons whose vocational or avocational pursuits bring them into contact with potentially rabid dogs, cats, foxes, skunks, bats, or other species at risk of having rabies should also be considered for pre-exposure prophylaxis.8
Vaccination is recommended for children living in or visiting countries where exposure to rabid animals is a constant threat. Worldwide statistics indicate children are more at risk than adults.
Pre-exposure prophylaxis is given for several reasons. First, it may provide protection to persons with inapparent exposure to rabies. Secondly, it may protect persons whose postexposure therapy might be expected to be delayed. Finally, although it does not eliminate the need for additional therapy after a rabies exposure, it simplifies therapy by eliminating the need for globulin and decreasing the number of doses of vaccine needed. This is of particular importance for persons at high risk of being exposed in countries where the available rabies immunizing products may carry a higher risk of adverse reactions.
Pre-exposure immunization does not eliminate the need for prompt prophylaxis following an exposure. It only reduces the postexposure treatment regimen.8
PRE-EXPOSURE RABIES TREATMENT GUIDE
1. Pre-exposure immunization
Consists of the three doses of HDCV, 1.0 mL, intramuscularly (deltoid area), one each on Days 0, 7 and 21 or 28. Administration of routine booster doses of vaccine depends on exposure risk category as noted in Table 1. Pre-exposure immunization of immunosuppressed persons is not recommended.8
| CRITERIA FOR PRE-EXPOSURE IMMUNIZATION | |||
|---|---|---|---|
| Risk category | Nature of risk | Typical populations | Pre-exposure regimen |
|
|||
| Continuous | Virus present continuously often in high concentrations. Aerosol, mucous membrane, bite or nonbite exposure possible. Specific exposures may go unrecognized. | Rabies research lab workers.* Rabies biologics production workers. | Primary pre-exposure immunization course. Serology every 6 months. Booster immunization when antibody titer falls below acceptable level.* |
| Frequent | Exposure usually episodic, with source recognized, but exposure may also be unrecognized. Aerosol, mucous membrane, bite or nonbite exposure. | Rabies diagnostic lab workers*, spelunkers, veterinarians, and animal control and wildlife workers in rabies epizootic areas. | Primary pre-exposure immunization course. Booster immunization or serology every 2 years.† |
| Infrequent (greater than population-at-large) | Exposure nearly always episodic with source recognized. Mucous membrane, bite or nonbite exposure. | Veterinarians and animal control and wildlife workers in areas of low rabies endemicity. Certain travelers to foreign rabies epizootic areas. Veterinary students. | Primary pre-exposure immunization course. No routine booster immunization or serology. |
| Rare (population-at-large) | Exposure always episodic, mucous membrane, or bite with source recognized. | US population-at-large, including individuals in rabies epizootic areas. | No pre-exposure immunization. |
B. Postexposure
See Table 2
The essential components of rabies postexposure prophylaxis are local treatment of wounds and immunization, including administration, in most instances, of both globulin and vaccine (Table 2).8,13
1. Local treatment of wounds
Immediate and thorough washing of all bite wounds and scratches with soap and water is perhaps the most effective measure for preventing rabies. In experimental animals, simple local wound cleansing has been shown to reduce markedly the likelihood of rabies.8,11
Tetanus prophylaxis and measures to control bacterial infection should be given as indicated.
2. Specific treatment
Postexposure antirabies immunization should always include administration of both antibody (preferably RIG) and vaccine, with one exception: persons who have been previously immunized with the recommended pre-exposure or postexposure regimens with HDCV or who have been immunized with other types of vaccines and have a history of documented adequate rabies antibody titer should receive only vaccine. The combination of globulin and vaccine is recommended for both bite exposures and nonbite exposures regardless of the interval between exposure and treatment.14,15 The sooner treatment is begun after exposure, the better. However, there have been instances in which the decision to begin treatment was made as late as 6 months or longer after the exposure due to delay in recognition that an exposure had occurred.8,13
3. Treatment outside the United States
If postexposure is begun outside the United States with locally produced biologics, it may be desirable to provide additional treatment when the patient reaches the US. State health departments should be contacted for specific advice in such cases.8
POSTEXPOSURE TREATMENT GUIDE
The following recommendations are only a guide. In applying them, take into account the animal species involved, the circumstances of the bite or other exposure, the vaccination status of the animal, and presence of rabies in the region. Local or state public health officials should be consulted if questions arise about the need for rabies prophylaxis.8
| Animal species | Condition of animal at time of attack | Treatment of exposed person* |
|---|---|---|
|
||
| DOMESTIC:
Dog and cat | Healthy and available for 10 days of observation. Rabid or suspected rabid. Unknown (escaped). | None unless animal develops rabies .† RIG‡ and HDCV. Consult public health officials. If treatment is indicated, give RIG‡ and HDCV. |
| WILD:
Skunk, bat, fox, coyote, raccoon, bobcat and other carnivores | Regard as rabid unless proven negative by laboratory tests.§ | RIG‡ and HDCV. |
| OTHER:
Livestock, rodents and lagomorphs (rabbits and hares) | Consider individually. Local and state public health officials should be consulted on questions about the need for rabies prophylaxis. Bites of squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, mice, other rodents, rabbits and hares, almost never call for antirabies prophylaxis. | |
CONTRAINDICATIONS
For postexposure treatment, there are no known specific contraindications to the use of Sanofi Imovax Rabies Vaccine. In cases of pre-exposure immunization, there are no known specific contraindications other than situations such as developing febrile illness, etc.
WARNINGS
Rabies Vaccine in this package is a unit dose to be delivered intramuscularly in the deltoid area.8
This vaccine must not be used intradermally or as a multiple dose dispensing unit. In both pre-exposure and postexposure immunization, the full 1.0 mL dose should be given intramuscularly.
In the case of pre-exposure immunization, recently a significant increase has been noted in "immune complex-like" reactions in persons receiving booster doses of HDCV.16 The illness characterized by onset 2-21 days post-booster, presents with a generalized urticaria and may also include arthralgia, arthritis, angioedema, nausea, vomiting, fever, and malaise. In no cases were the illnesses life-threatening. Preliminary data suggest this "immune complex-like" illness may occur in up to 6% of persons receiving booster vaccines and much less frequently in persons receiving primary immunization. Additional experience with this vaccine is needed to define more clearly the risk of these adverse reactions.8,17
Two cases of neurologic illness resembling Guillain-Barré syndrome,18,19 a transient neuroparalytic illness, that resolved without sequelae in 12 weeks and a focal subacute central nervous system disorder temporally associated with HDCV, have been reported.20
All serious systemic neuroparalytic or anaphylactic reactions to a rabies vaccine should be immediately reported to the state health department or Sanofi Pasteur Inc., 1-800-VACCINE (1-800-822-2463).8
PRECAUTIONS
IN ADULTS AND CHILDREN THE VACCINE SHOULD BE INJECTED INTO THE DELTOID MUSCLE. IN INFANTS AND SMALL CHILDREN, THE MID-LATERAL ASPECT OF THE THIGH MAY BE PREFERABLE.
General
When a person with a history of hypersensitivity must be given rabies vaccine, antihistamines may be given; epinephrine (1:1000) should be readily available to counteract anaphylactic reactions, and the person should be carefully observed after immunization.
While the concentration of antibiotics in each dose of vaccine is extremely small, persons with known hypersensitivity to any of these agents could manifest an allergic reaction. While the risk is small, it should be weighed in light of the potential risk of contracting rabies.
Drug interactions
Corticosteroids, other immunosuppressive agents, and immunosuppressive illnesses can interfere with the development of active immunity and predispose the patient to developing rabies. Immunosuppressive agents should not be administered during postexposure therapy, unless essential for the treatment of other conditions. When rabies postexposure prophylaxis is administered to persons receiving steroids or other immunosuppressive therapy, it is especially important that serum be tested for rabies antibody to ensure that an adequate response has developed.8
Usage in pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with Imovax Rabies Vaccine. It is also not known whether the product can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Rabies vaccine should be given to a pregnant woman only if clearly needed.
Because of the potential consequences of inadequately treated rabies exposure and limited data that indicate that fetal abnormalities have not been associated with rabies vaccination, pregnancy is not considered a contraindication to postexposure prophylaxis.8,21 If there is substantial risk of exposure to rabies, pre-exposure prophylaxis may also be indicated during pregnancy.8
ADVERSE REACTIONS
ALSO SEE WARNINGS AND CONTRAINDICATIONS SECTIONS FOR ADDITIONAL STATEMENTS.
Once initiated, rabies prophylaxis should not be interrupted or discontinued because of local or mild systemic adverse reactions to rabies vaccine. Usually such reactions can be successfully managed with anti-inflammatory and antipyretic agents (eg, aspirin).
Reactions after vaccination with HDCV are less common than with previously available vaccines.12,16,17 In a study using five doses of HDCV, local reactions, such as pain, erythema, and swelling or itching at the injection site were reported in about 25% of recipients of HDCV, and mild systemic reactions such as headache, nausea, abdominal pain, muscle aches and dizziness were reported in about 20% of recipients.8
Serious systemic anaphylactic or neuroparalytic reactions occurring during the administration of rabies vaccines pose a dilemma for the attending physician. A patient's risk of developing rabies must be carefully considered before deciding to discontinue vaccination. Moreover, the use of corticosteroids to treat life-threatening neuroparalytic reactions carries the risk of inhibiting the development of active immunity to rabies. It is especially important in these cases that the serum of the patient be tested for rabies antibodies. Advice and assistance on the management of serious adverse reactions in persons receiving rabies vaccines may be sought from the state health department.8
DOSAGE AND ADMINISTRATION
Before administration, parenteral drug products should be checked visually for any deviation from normal appearance including container integrity. The syringe and its package should also be inspected prior to use for evidence of leakage, premature activation of the plunger, or a faulty tip seal. If evidence of such defects are observed, the syringe should not be used.
The package contains a vial of freeze-dried vaccine, a syringe containing 1.0 mL of diluent, a plunger for the syringe, and a needle for reconstitution. Attach the plunger and reconstitution needle to the syringe and reconstitute the freeze-dried vaccine by injecting the diluent into the vaccine vial. Gently swirl the contents until completely dissolved and withdraw the total contents of the vial into the syringe. Remove the reconstitution needle and discard. For administration, use a needle of your choice that is suitable for intramuscular injection of your patient.
The syringe is intended for single use only, must not be reused, and must be disposed of properly and promptly following its use.
To help avoid HIV (AIDS), HBV (Hepatitis), and other infectious diseases due to accidental needlesticks, contaminated needles should not be recapped or removed, unless there is no alternative or that such action is required by a specific medical procedure.
The reconstituted vaccine should be used immediately.
After preparation of the injection site, immediately inject the vaccine intramuscularly. For adults and children, the vaccine should be injected into the deltoid muscle.22-27,29 In infants and small children, the mid lateral aspect of the thigh may be preferable. Care should be taken to avoid injection into or near blood vessels and nerves. If blood or any suspicious discoloration appears in the syringe, do not inject but discard contents and repeat procedure using a new dose of vaccine, at a different site.
NOTE: The freeze-dried vaccine is creamy white to orange. After reconstitution, it is pink to red.
A. Pre-exposure dosage
1. Primary vaccination
In the United States, the Immunization Practices Advisory Committee (ACIP) recommends three injections of 1.0 mL each, one injection on Day 0 and one on Day 7 and one either on Day 21 or 28.8
2. Booster dose
Persons working with live rabies virus in research laboratories and in vaccine production facilities should have rabies antibody titers checked every six months and boosters given as needed to maintain an adequate titer. (For definition of adequate titer, see CLINICAL PHARMACOLOGY.) Only laboratory workers, such as those doing rabies diagnostic tests, spelunkers and veterinarians, animal control and wildlife officers in areas where rabies is epizootic should have boosters every 2 years or have their serum tested for rabies antibody every 2 years and, if the titer is inadequate, have a booster dose. Veterinarians and animal control and wildlife officers, if working in areas of low rabies endemicity, do not require routine booster doses of HDCV after completion of primary pre-exposure immunization (Table 1.)8
Persons who have experienced "immune complex-like" hypersensitivity reactions should receive no further doses of HDCV unless they are exposed to rabies or they are truly likely to be inapparently and/or unavoidably exposed to rabies virus and have unsatisfactory antibody titers.
B. Postexposure dosage
The World Health Organization established a recommendation for six intramuscular doses of human diploid cell vaccine (HDCV) based on studies in Germany and Iran.3,7 Used in this way, a total of 6 injections of a 1.0 mL dose of vaccine are given according to the following schedule: on Day 0, 3, 7, 14, 30 and 90. The first dose should be accompanied by Rabies Immune Globulin (RIG) or Antirabies Serum (ARS). If possible, up to half the dose of RIG or ARS should be used to infiltrate the wound, and the rest administered intramuscularly, in a different site from the rabies vaccine, preferably in the gluteal region.
Studies conducted at the CDC in the United States have shown that a regimen of 1 dose of Rabies Immune Globulin (RIG) and 5 doses of HDCV induced an excellent antibody response in all recipients. Of 511 persons bitten by proven rabid animals and so treated, none developed rabies.8
Based on these data, the ACIP recommends a 5-dose regimen for postexposure situations. Five 1.0 mL doses are given intramuscularly on Day 0, 3, 7, 14 and 28 in conjunction with RIG on Day 0.8
Because the antibody response following the recommended vaccination regimen with HDCV has been so satisfactory, routine postvaccination serologic testing is not recommended. Serologic testing is indicated in unusual circumstances, as when the patient is known to be immunosuppressed. Contact state health department or CDC for recommendations.8,28
C. Postexposure therapy of previously immunized persons
When an immunized person who was vaccinated by the recommended regimen with a cell culture vaccine or who had previously demonstrated rabies antibody is exposed to rabies, that person should receive two intramuscular doses (1.0 mL each) of HDCV, one immediately and one 3 days later. RIG should not be given in these cases. If the immune status of a previously vaccinated person who did not receive the recommended HDCV regimen is not known, full primary postexposure antirabies treatment (RIG plus 5 doses of HDCV) may be necessary. In such cases, if antibody can be demonstrated in a serum sample collected before vaccine is given, treatment can be discontinued after at least two doses of HDCV.8
HOW SUPPLIED
IMOVAX RABIES VACCINE is supplied in a tamper evident unit dose box with:
- -
- One vial of freeze-dried vaccine containing a single dose.
- -
- One syringe containing diluent. A separate plunger is provided for insertion and use.
- -
- One disposable needle for reconstitution.
Product No. 49281-250-51
CPT® Code: 90675
CPT is a registered trademark of the American Medical Association.
REFERENCES
- Aoki FY, Tyrell DAJ, Hill LE. Immunogenicity and acceptability of a human diploid cell culture rabies vaccine in volunteers. The Lancet, March 22, pp. 660-2 (1975).
- Cox JH, Schneider LG. Prophylactic immunization of humans against rabies by intradermal inoculation of human diploid cell culture vaccine. J Clin Microbiol 3: 96-101 (1976).
- Kuwert EK, Marcus I, Werner J, Iwand A, Thraenhart O. Some experiences with human diploid cell strain—(HDCS) rabies vaccine in pre-and postexposure vaccinated humans. Develop Biol Standard 40: 79-88 (1978).
- Ajjan N, Soulebot J-P, Stellmann C, Biron G, Charbonnier C, Triau R, Mérieux C. Résultats de la vaccination antirabique préventive par le vaccin inactivé concentré souche rabies PM/W138-1503-3M cultivés sur cellules diploïdes humaines. Develop Biol Standard 40:89-199 (1978).
- Costy-Berger F. Vaccination antirabique préventive par du vaccin préparé sur cellules diploïdes humaines. Develop Biol Standard 40: 101-4 (1978).
- Bernard KW, Roberts MA, Sumner J, Winkler WG, Mallonee J, Baer GM, Chaney R. Human diploid cell rabies vaccine JAMA 247:1138-42 (1982).
- Bahmanyar M, Fayaz A, Nour-Salehi S, Mohammadi M, Koprowski H. Successful protection of humans exposed to rabies infection. JAMA 236: 2751-4 (1976).
- CDC. Recommendations of the Immunization Practices Advisory Committee (ACIP). Rabies Prevention—United States, 1984, MMWR 33: 393-402, 407-8 (1984).
- Anderson LJ, Nicholson KG, Tauxe RV, Winkler WG. Human rabies in the United States, 1960 to 1979, epidemiology, diagnosis and prevention. Ann Intern Med 100: 728-35 (1984).
- WHO. Sixth report of the Expert Committee on Rabies. Geneva Switzerland: World Health Organization. (WHO technical report No. 523) (1973).
- Baer GM, ed. The natural history of rabies. New York: Academic Press. (1975).
- Greenberg M, Childress J. Vaccination against rabies with duck-embryo and Semple vaccines. JAMA 173: 333-7 (1960).
- Helmick CG. The epidemiology of human rabies postexposure prophylaxis. JAMA 250:1990-6 (1983).
- Devriendt J, Staroukine M, Costy F, Vanderhaegen, J-J. Fatal encephalitis apparently due to rabies. JAMA 248: 2304-6 (1982).
- CDC. Human Rabies—Rwanda. MMWR 31:135 (1982).
- CDC. Systemic allergic reactions following immunization with human diploid cell rabies vaccine. MMWR 33: 185-7 (1984).
- Rubin RH, Hattwick MAW, Jones S, Gregg MB, Schwartz VD. Adverse reactions to duck embryo rabies vaccine. Ann Intern Med 78: 643-9 (1973).
- Boe E, Nyland H. Guillain-Barré syndrome after vaccination with human diploid cell rabies vaccine. Scand J Infect Dis 12: 231-2 (1980).
- CDC. Adverse reactions to human diploid cell rabies vaccine. MMWR 29: 609-10 (1980).
- Bernard KW, Smith PW, Kader FJ, Moran MJ. Neuroparalytic illness and human diploid cell rabies vaccine. JAMA 248: 3136-8 (1982).
- Varner MW, McGuinness GA, Galask RP. Rabies vaccination in pregnancy. Am J of Obst and Gyn 143:717-18 (1982).
- Cockshott WP, Thompson GT, Howlett LJ, Seely ET. Intramuscular or intralipomatous injections? N Eng J Med 307: 356-58 (1982).
- CDC. General Recommendations on Immunization, ACIP. MMWR 32: 1-8, 13-17 (1983).
- Committee on Immunization Council of Medical Societies, American College of Physicians. Guide for Adult Immunizations. (1985).
- CDC. Rabies postexposure prophylaxis with HDCV: Lower neutralizing antibody titers with Wyeth vaccine. MMWR 34: 90-92 (1985).
- Shill M, Baynes RD, Miller SD. Fatal rabies encephalitis despite appropriate postexposure prophylaxis. N Engl J Med 316: 1257-58 (1987).
- Baer GM, Fishbein DB. Rabies postexposure prophylaxis. N Engl J Med 316: 1270-72 (1987).
- CDC. Recommendations of the Immunization Practices Advisory Committee (ACIP). Supplementary statement on rabies vaccine and serologic testing. MMWR 30: 535-6 (1981).
- CDC. Human rabies despite treatment with Rabies Immune Globulin and Human Diploid Cell Rabies Vaccine - Thailand. MMWR 36: 759-765 (1987).
Product information as of December 2005
US Govt License #1724
Manufactured by:
Sanofi Pasteur SA
Lyon France
Distributed by:
Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
1-800-VACCINE (1-800-822-2463)
94234-0
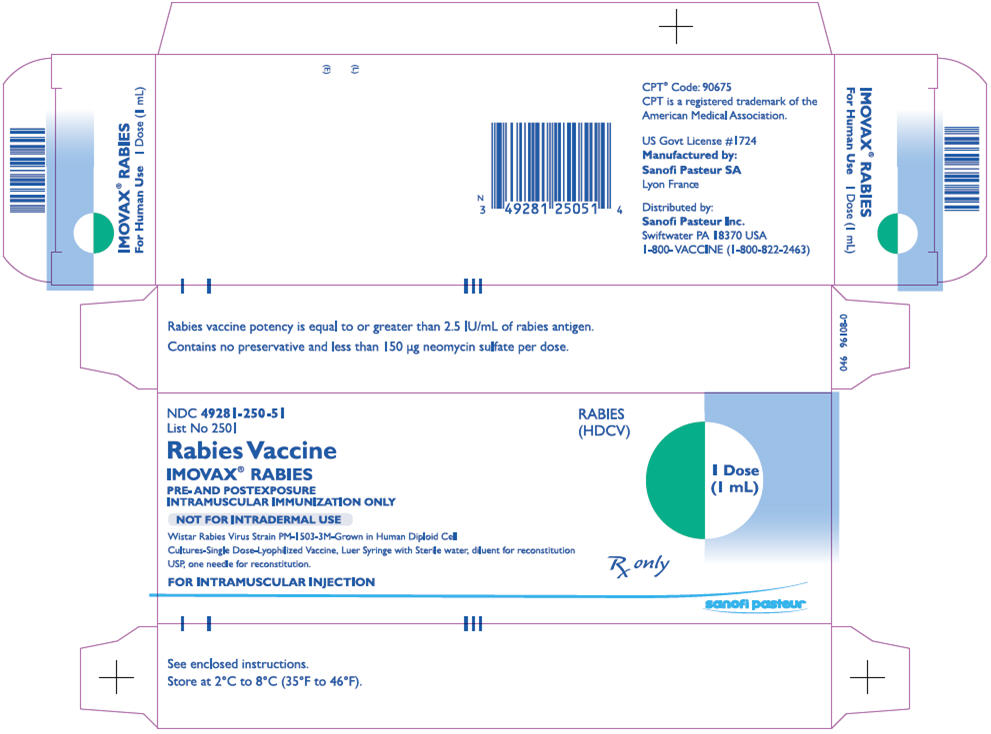
PRINCIPAL DISPLAY PANEL - 1 mL Vial Label
NDC 49281-250-51
Rabies
Vaccine
IMOVAX® RABIES
RABIES
(HDCV)
1 mL
IM only
Rx only
Mfd by: Sanofi Pasteur SA
Lyon France US Govt Lic #1724
Dist by: Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
1-800-VACCINE (1-800-822-2463)

PRINCIPAL DISPLAY PANEL - 1 mL Syringe Label
NDC 49281-249-01
1 mL
Sterile Water,
diluent for
reconstitution
USP
Rx only
Contains no preservative
Sanofi Pasteur

PRINCIPAL DISPLAY PANEL - 1 mL Kit Carton
NDC 49281-250-51
List No 2501
Rabies Vaccine
IMOVAX® RABIES
PRE- AND POSTEXPOSURE
INTRAMUSCULAR IMMUNIZATION ONLY
NOT FOR INTRADERMAL USE
Wistar Rabies Virus Strain PM-1503-3M-Grown in Human Diploid Cell
Cultures-Single Dose-Lyophilized Vaccine, Luer Syringe with Sterile water, diluent for reconstitution
USP, one needle for reconstitution.
FOR INTRAMUSCULAR INJECTION
RABIES
(HDCV)
1 Dose
(1 mL)
Rx only
sanofi pasteur
| IMOVAX RABIES
rabies virus strain pm-1503-3m antigen (propiolactone inactivated) and water kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103931 | 06/09/1980 | |
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sanofi Pasteur SA | 381505171 | MANUFACTURE | |
Revised: 06/2012 Sanofi Pasteur Inc.