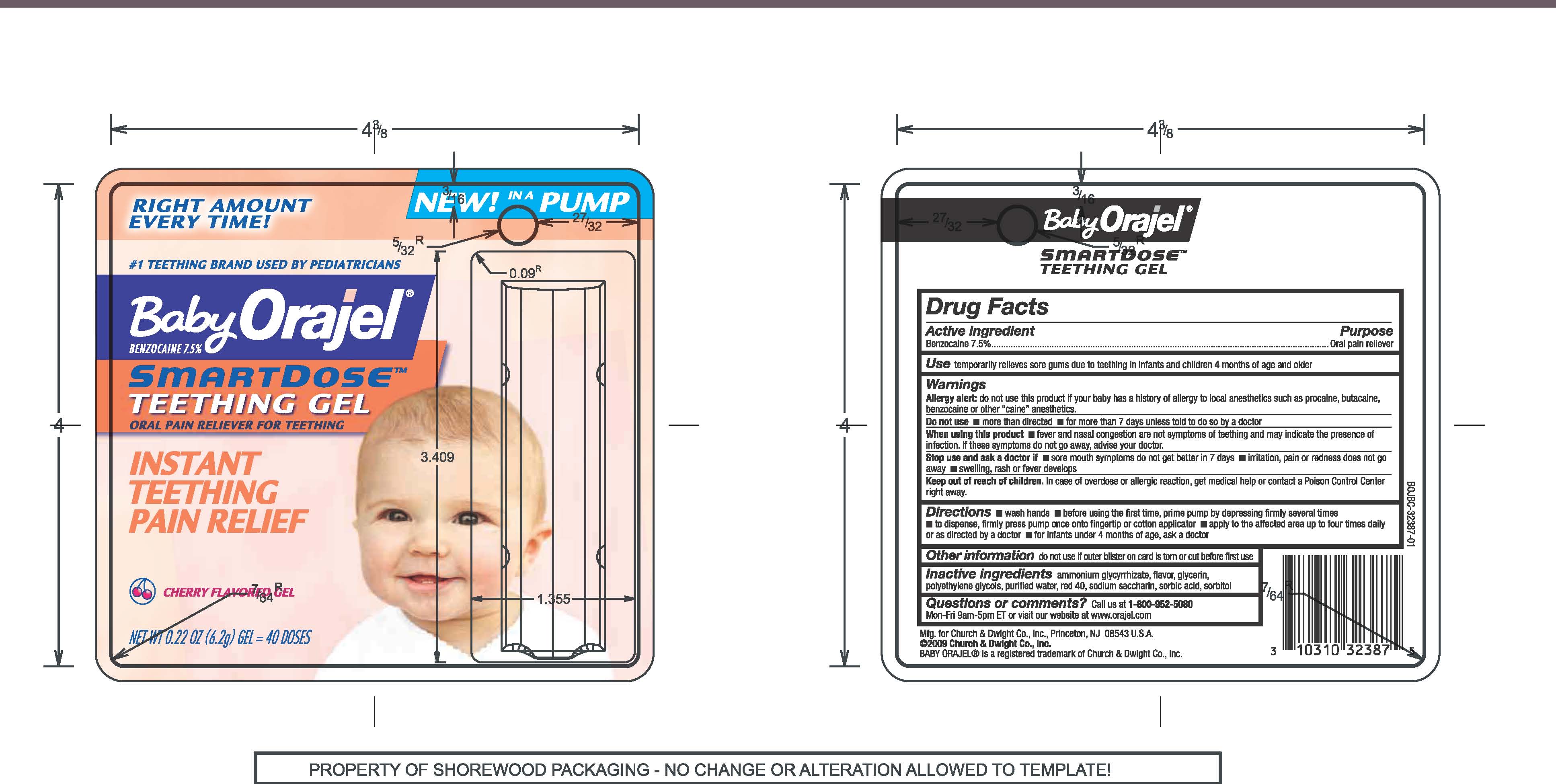
BABY ORAJEL SMARTDOSE TEETHING
-
benzocaine gel
Church & Dwight Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Use for the temporary relief of sore gums due to teething in infants and children 4 months of age or older
Allergy Alert
do not use this product if our baby has a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
Do not use
more than directed, for more than 7 days unless told to do so by a doctor
When using this product fever and nasal congestion are not symptoms of teething and may indicate the presence of infection. If these symptoms do not go away, advise your doctor.
Stop use and ask a dentist of doctor if sore mouth symptoms do not get better in 7 days, irritation, pain or redness does not go away, swelling, rash or fever develops.
Directions
wash hands,
before using the first time, prime pump by depressing firmly several times
to dispsnse, firmly press pump once onto fingertip or cotton applicator
apply to the affected area up to four times daily or as directed by doctor,
for infants under 4 months of age, ask a dentist or doctor
Other information
do not use if outer blister on card is torn or cut before first use
Inactive ingredients
ammonium glycyrrhizate, flavor, glycerin, polyethylene glycols, purified water, red 40, sodium saccharin, sorbic acid, sorbitol
Questions or comments? call us at 1-800-952-5080 Mon-Fri 9am-5pm ET or visit our website at www.orajel.com
Keep out of reach of children. in case of overdose or allergic reaction, get medical help or contact a Poison Control Center right away.
| BABY ORAJEL SMARTDOSE TEETHING
benzocaine gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part356 | 07/22/2010 | 04/24/2012 |
| Labeler - Church & Dwight Co., Inc. (001211952) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Accupac | 071609663 | manufacture | |
Revised: 07/2010 Church & Dwight Co., Inc.