carisoprodol (Carisoprodol) tablet
[Actavis Totowa LLC]
DESCRIPTION
Carisoprodol is a white, crystalline powder, having a mild, characteristic odor and a bitter taste. Carisoprodol is N-isopropyl-2-methyl-2-propyl-1, 3-propanediol dicarbamate and its molecular weight is 260.34. Its structural formula is as follows:
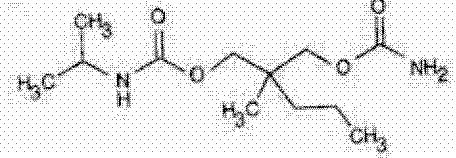
C12H24N2O4 MW 260.34
Each tablet for oral administration contains 350 mg carisoprodol. In addition, each tablet contains the following inactive ingredients: hydroxypropyl methylcellulose, lactose monohydrate, microcrystalline cellulose, sodium starch glycolate, stearic acid and talc.
CLINICAL PHARMACOLOGY
Carisoprodol produces muscle relaxation in animals by blocking interneuronal activity in the descending reticular formation and spinal cord. The onset of action is rapid and effects last four to six hours.
INDICATIONS AND USAGE
Carisoprodol is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Carisoprodol does not directly relax tense skeletal muscles in man.
CONTRAINDICATIONS
Acute intermittent porphyria as well as allergic or idiosyncratic reactions to carisoprodol or related compounds such as meprobamate, mebutamate, or tybamate.
WARNINGS
Idiosyncratic Reactions: On very rare occasions, the first dose of carisoprodol has been followed by idiosyncratic symptoms appearing within minutes or hours. Symptoms reported include: extreme weakness, transient quadriplegia, dizziness, ataxia, temporary loss of vision, diplopia, mydriasis, dysarthria, agitation, euphoria, confusion, and disorientation. Symptoms usually subside over the course of the next several hours. Supportive and symptomatic therapy, including hospitalization, may be necessary.
Usage in Pregnancy and Lactation: Safe usage of this drug in pregnancy or lactation has not been established. Therefore, use of this drug in pregnancy, in nursing mothers, or in women of childbearing potential requires that the potential benefits of the drug be weighed against the potential hazards to mother and child. Carisoprodol is present in breast milk of lactating mothers at concentrations two to four times that of maternal plasma. This factor should be taken into account when use of the drug is contemplated in breast-feeding patients.
Usage in children: Because of limited clinical experience, Carisoprodol is not recommended for use in patients under 12 years of age.
Potentially Hazardous Tasks: Patients should be warned that this drug may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a motor vehicle or operating machinery.
Additive Effects: Since the effects of carisoprodol and alcohol or carisoprodol and other CNS depressants or psychotropic drugs may be additive, appropriate caution should be exercised with patients who take more than one of these agents simultaneously.
Drug Dependence: In dogs, no withdrawal symptoms occurred after abrupt cessation of carisoprodol from dosages as high as 1 gm/kg/day. In a study in man, abrupt cessation of 100 mg/kg/day (about five times the recommended daily adult dosage) was followed in some subjects by mild withdrawal symptoms such as abdominal cramps, insomnia, chilliness, headache, and nausea. Delirium and convulsions did not occur. In clinical use, psychological dependence and abuse have been rare, and there have been no reports of significant abstinence signs. Nevertheless, the drug should be used with caution in addiction-prone individuals.
PRECAUTIONS
Carisoprodol is metabolized in the liver and excreted by the kidney; to avoid its excess accumulation, caution should be exercised in administration to patients with compromised liver or kidney function.
ADVERSE REACTIONS
Central Nervous System - Drowsiness and other CNS effects may require dosage reduction. Also observed: dizziness, vertigo, ataxia, tremor, agitation, irritability, headache, depressive reactions, syncope, and insomnia. (See also Idiosyncratic Reactions under " Warnings").
Allergic or Idiosyncratic - Allergic or idiosyncratic reactions occasionally develop. They are usually seen within the period of the first to fourth dose in patients having had no previous contact with the drug. Skin rash, erythema multiforme, pruritus, eosinophilia, and fixed drug eruption with cross reaction to meprobamate have been reported with carisoprodol. Severe reactions have been manifested by asthmatic episodes, fever, weakness, dizziness, angioneurotic edema, smarting eyes, hypotension, and anaphylactoid shock. (See also Idiosyncratic Reactions under " Warnings").
In case of allergic or idiosyncratic reactions to carisoprodol, discontinue the drug and initiate appropriate symptomatic therapy, which may include epinephrine, antihistamines, and in severe cases corticosteroids. In evaluating possible allergic reactions, also consider allergy to excipients (information on excipients is available to physicians on request).
Cardiovascular - Tachycardia, postural hypotension, and facial flushing.
Gastrointestinal - Nausea, vomiting, hiccup, and epigastric distress.
Hematologic - Leukopenia, in which other drugs or viral infection may have been responsible, and pancytopenia, attributed to phenylbutazone, have been reported. No serious blood dyscrasias have been attributed to carisoprodol.
OVERDOSAGE
Overdosage of carisoprodol has produced stupor, coma, shock, respiratory depression, and, very rarely, death. The effects of an overdosage of carisoprodol and alcohol or other CNS depressants or psychotropic agents can be additive even when one of the drugs has been taken in the usual recommended dosage. Any drug remaining in the stomach should be removed and symptomatic therapy given. Should respiration or blood pressure become compromised, respiratory assistance, central nervous system stimulants, and pressor agents should be administered cautiously as indicated. Carisoprodol is metabolized in the liver and excreted by the kidney. Although carisoprodol overdosage experience is limited, the following types of treatment have been used successfully with the related drug meprobamate: diuresis, osmotic (mannitol) diuresis, peritoneal dialysis, and hemodialysis (carisoprodol is dialyzable). Careful monitoring of urinary output is necessary and caution should be taken to avoid overhydration. Observe for possible relapse due to incomplete gastric emptying and delayed absorption. Carisoprodol can be measured in biological fluids by gas chromatography (Douglas, J.F. et al: J Pharm Sci 58: 145, 1969)
DOSAGE AND ADMINISTRATION:
The usual adult dosage of carisoprodol tablets is one 350 mg tablet, three times daily and at bedtime. Usage in patients under 12 is not recommended.
HOW SUPPLIED
Carisoprodol Tablets, USP 350 mg: White, round, unscored tablets, debossed with A136 on one side, are available in bottles of 100's, 500's and 1000's
Store at controlled room temperature 15°- 30°C (59° - 86°F).
Dispense in a tight, light-resistant container as defined in the USP.
Manufactured by: Actavis Totowa LLC
990 Riverview Drive, Totowa, NJ 07512 USA
7850-02
11/06
| Carisoprodol (Carisoprodol) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
Revised: 05/2008Actavis Totowa LLC