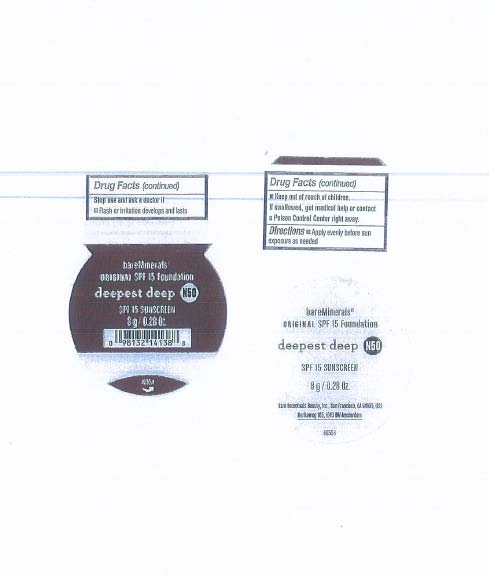
DEEPEST DEEP
-
zinc oxide powder
Bare Escentuals Beauty Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
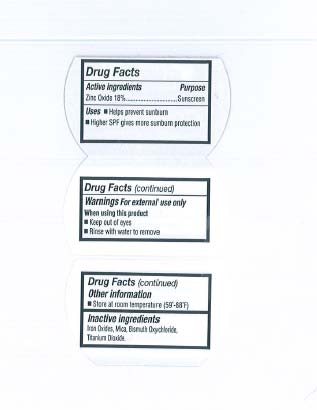
Active Ingredients
Zinc Oxide 18%
- Helps prevent sunburn
- Higher SPF gives more sunburn protection
Warnings
For External use only
When using this product
- Keep out of eyes
- Rinse with water to remove
Stop use and ask a doctor if
Rash or irritation develops and lasts
Keep out of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply evenly before sun exposure as needed
DEEPEST DEEP
zinc oxide powder |
|
|
|
|
|
|
|
|
|
|
Revised: 06/2011 Bare Escentuals Beauty Inc.