LOHIST PD
-
brompheniramine maleate and
pseudoephedrine hydrochloride solution/ drops
Larken Laboratories, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION
LoHist PD Pediatric Drops is a cherry flavored, clear liquid antihistamine/decongestant.
Each dropperful (1 mL) for oral administration contains:
Brompheniramine Maleate . . . . . . . . . . . . . . . . . . . . . . 1 mg
Pseudoephedrine HCl . . . . . . . . . . . . . . . . . . . . . . . . 12.5 mg
Inactive Ingredients: Cherry flavor, citric acid, glycerin, sodium benzoate, propylene glycol, purified water, sodium saccharin, and sorbitol.
Brompheniramine Maleate is an antihistamine with the chemical name 2-Pyridinepropanamine, γ-(4-bromophenyl)-N,N-dimethyl-, (±)-, (Z) -2-butenedioate (1:1). Its structure is as follows:
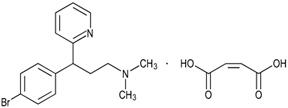
C16H19BrN2 • C4H4O4 M.W. 435.31
Pseudoephedrine Hydrochloride is a nasal decongestant with the chemical name:
benzenemethanol, α-[1-(methylamino)ethyl]-, [S-(R*,R*)]-, hydrochloride. Its structure is as follows:
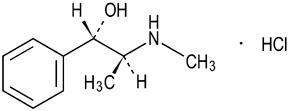
C10H15NO • HCl M.W. 201.69
CLINICAL PHARMACOLOGY
Brompheniramine maleate is a histamine antagonist, specifically an H1-receptor-blocking agent belonging to the alkylamine class of antihistamines. Antihistamines appear to compete with histamine for receptor sites on effector cells. Brompheniramine also has anticholinergic (drying) and sedative effects. Among the antihistaminic effects, it antagonizes the allergic response (vasodilatation, increased vascular permeability, increased mucus secretion) of nasal tissue. Brompheniramine is well absorbed from the gastrointestinal tract, with peak plasma concentration after single, oral doses of 4 mg reached in 5 hours; urinary excretion is the major route of elimination, mostly as products of biodegradation; the liver is assumed to be the main site of metabolic transformation.
Pseudoephedrine hydrochloride is an orally effective nasal decongestant that acts on alpha-adrenergic receptors in the mucosa of the respiratory tract producing vasoconstriction. Pseudoephedrine shrinks swollen nasal mucous membranes, reduces tissue hyperemia, edema, and nasal congestion and increases nasal airway patency. Eustachian ostia may be opened. Pseudoephedrine produces little if any rebound congestion.
INDICATIONS AND USAGE
LoHist PD is indicated for the temporary relief of nasal congestion and pressure, runny nose, sneezing, itching of the nose or throat, and itchy watery eyes, due to hay fever, or other upper respiratory allergies (allergic rhinitis).
CONTRAINDICATIONS
LoHist PD is contraindicated in patients with hypersensitivity or idiosyncrasy to any of its ingredients, patients taking monoamine oxidase (MAO) inhibitors or for two weeks after stopping the MAOI drug (see PRECAUTIONS, Drug Interactions section), patients with narrow-angle glaucoma, urinary retention, peptic ulcer, severe hypertension, severe coronary artery disease, patients with breathing problems such as emphysema or chronic bronchitis, or nursing mothers. Antihistamines should not be used to treat lower respiratory tract conditions including asthma.
WARNINGS
Do not exceed recommended dosage. If nervousness, dizziness, or sleeplessness occur, discontinue use and consult a physician. If symptoms do not improve within 7 days or are accompanied by fever, consult a physician.
Sympathomimetic amines should be used judiciously and sparingly in patients with hypertension, diabetes mellitus, ischemic heart disease, increased intraocular pressure, hyperthyroidism or prostatic hypertrophy. Sympathomimetics may produce central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension.
Antihistamines may impair mental and physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery, and may impair mental alertness in children. In the young child, they may produce excitation.
PRECAUTIONS
General
Because of its antihistamine component, LoHist PD should be used with caution in patients with a history of bronchial asthma, narrow angle glaucoma, gastrointestinal obstruction, or urinary bladder neck obstruction. Because of its sympathomimetic component, this syrup should be used with caution in patients with diabetes, hypertension, heart disease, or thyroid disease.
Information for Patients
Antihistamines may cause drowsiness and ambulatory patients who operate machinery or motor vehicles should be cautioned accordingly.
Drug Interactions
MAO inhibitors and beta adrenergic blockers increase the effect of sympathomimetics. Sympathomimetics may reduce the antihypertensive effects of methyldopa, mecamylamine, reserpine and veratrum alkaloids. Concomitant use of antihistamines with alcohol, tricyclic antidepressants, barbiturates and other CNS depressants may have an additive effect.
Pregnancy
Pregnancy Category C: Animal reproduction studies have not been conducted with LoHist PD. It is also not known whether LoHist PD can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. LoHist PD should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from LoHist PD, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
Hyperreactive individuals may display ephedrine-like reactions such as tachycardia, palpitations, headache, dizziness, or nausea. Patients sensitive to antihistamines may experience mild sedation. Sympathomimetic drugs have been associated with certain untoward reactions including fear, anxiety, tenseness, restlessness, tremor, weakness, pallor, respiratory difficulty, dysuria, insomnia, hallucinations, convulsions, CNS depression, arrhythmias, and cardiovascular collapse with hypotension.
Possible side effects of antihistamines are drowsiness, restlessness, dizziness, weakness, dry mouth, anorexia, nausea, headache, nervousness, blurring of vision, heartburn, dysuria and very rarely dermatitis. Patient idiosyncrasy to adrenergic agents may be manifested by insomnia, dizziness, weakness, tremor or arrhythmias.
OVERDOSAGE
No information is available as to specific results of an overdose of LoHist PD. The signs, symptoms and treatment described below are those of H1 antihistamines, and ephedrine overdose.
Symptoms: Should antihistamine effects predominate, central action constitutes the greatest danger. In the small child, symptoms include excitation, hallucination, ataxia, incoordination, tremors, flushed face and fever. Convulsions, fixed and dilated pupils, coma and death may occur in severe cases. In the adult, fever and flushing are uncommon; excitement leading to convulsion and postictal depression is often preceded by drowsiness and coma. Respiration is usually not seriously depressed; blood pressure is usually stable.
Should sympathomimetic symptoms predominate, central effects include restlessness, dizziness, tremor, hyperactive reflexes, talkativeness, irritability and insomnia. Cardiovascular and renal effects include difficulty in micturition, headache, flushing, palpitation, cardiac arrhythmia, hypertension with subsequent hypotension and circulatory collapse. Gastrointestinal effects include dry mouth, metallic taste, anorexia, nausea, vomiting, diarrhea and abdominal cramps.
Treatment: The stomach should be emptied promptly by emetics and/or gastric lavage. The installation of activated charcoal also should be considered. Cardiac function and serum electrolytes should be monitored and treatment initiated if indicated. If convulsions or marked CNS excitement occurs, diazepam may be used.
DOSAGE AND ADMINISTRATION
| AGE | DOSE |
| Children 6 to under 12 years of age | 2 dropperfuls (2 mL) every 4-6 hours |
| Children 2 to under 6 years of age | 1 dropperful (1 mL) every 4-6 hours |
| Children under 2 | As directed by a physician |
HOW SUPPLIED
LoHist PD Pediatric Drops is supplied in bottles of 1 fl. oz. (30 mL), NDC 68047-011-30. Calibrated, shatter-proof dropper enclosed in each carton.
Storage and Handling
Store at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F). [See USP Controlled Room Temperature.]
LoHist PD contains safety seal under bottle cap. Pharmacist, dispense in original container.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSAGE, CONTACT A POISON CONTROL CENTER AND SEEK PROFESSIONAL ASSISTANCE IMMEDIATELY.
Rx only
Distributed by:
LARKEN LABORATORIES, INC.
Canton, MS 39046
Rev. 10/08
| LOHIST
PD
brompheniramine maleate 1 mg/ pseudoephedrine hcl 12.5 mg/ 1ml solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 04/27/2005 | 11/30/2011 | |
| Labeler - Larken Laboratories, Inc. (791043719) |
| Registrant - Larken Laboratories, Inc. (791043719) |
Revised: 04/2012 Larken Laboratories, Inc.

